P35405
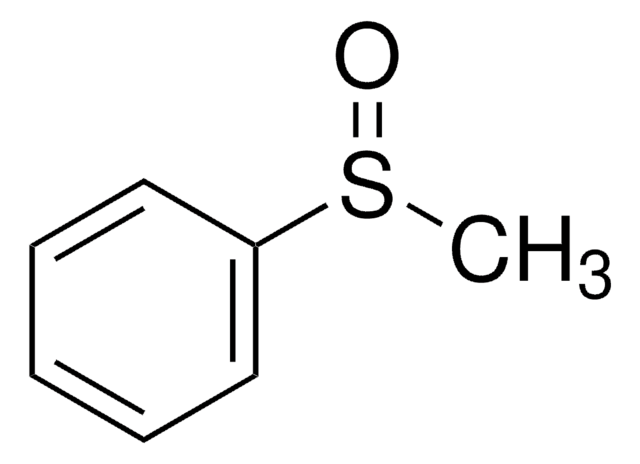
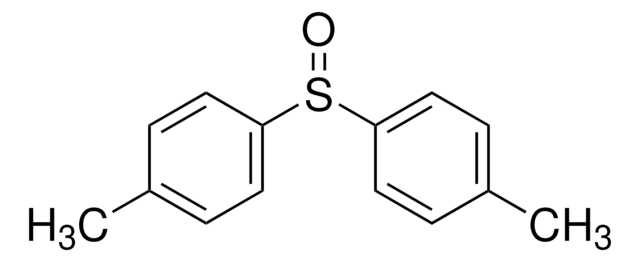
Diphenyl sulfoxide
96%
Synonym(s):
Phenyl sulfoxide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(C6H5)2SO
CAS Number:
Molecular Weight:
202.27
Beilstein:
1908444
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
form
crystals
bp
206-208 °C/13 mmHg (lit.)
mp
69-71 °C (lit.)
SMILES string
O=S(c1ccccc1)c2ccccc2
InChI
1S/C12H10OS/c13-14(11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10H
InChI key
JJHHIJFTHRNPIK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Preparation of radiochemicals: Diphenyl sulfoxide plays a role in the synthesis of [(11)C]cyanide from [(11)C]methyl iodide, facilitating rapid and efficient production of radiochemicals for medical imaging applications (Kikuchi et al., 2022).
- Catalytic oxidation processes: The photocatalytic and catalytic oxidation of diphenyl sulphide to sulfoxide and sulfone was examined, highlighting the effectiveness of hydrogen peroxide and TiO2 polymorphs in optimizing chemical processes (Mikrut et al., 2022).
- Dielectric properties research: The study on dielectric properties of high organic sulfur coal highlighted the modeling of sulfur compounds, which could include diphenyl sulfoxide, enhancing our understanding of materials science in energy sectors (Cai et al., 2019).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
S Yoshihara et al.
Archives of biochemistry and biophysics, 249(1), 8-14 (1986-08-15)
To characterize the properties of diphenyl sulfoxide (DPSO) as a new type of electron acceptor for guinea pig liver aldehyde oxidase (AO), we compared the kinetics of the reductions of DPSO and other classical electron acceptors such as O2 and
Wen-xian Li et al.
Guang pu xue yu guang pu fen xi = Guang pu, 22(6), 905-907 (2003-08-14)
(Tb1-x Tmx).L2.(ClO4).2H2O(x = 0.000 to 0.200, L = C6H5SOCH2COO-) have been synthesized. The coordination compounds have been studied by means of composition analysis, molar conductivity, IR, and the condition of coordination have been inferred. In the fluorescent spectra it was
J A Kozlowski et al.
Bioorganic & medicinal chemistry letters, 10(20), 2255-2257 (2000-10-31)
Structure activity studies on [4-(phenylsulfonyl)phenyl]methylpiperazine led to the discovery of 4-cyclohexyl-alpha-[4-[[4-methoxyphenyl(S)-sufinyl]phenyl]-1-pi perazineacetonitrile, 1, an M2 selective muscarinic antagonist. Affinity at the cloned human M2 receptor was 2.7 nM; the M1/M2 selectivity is 40-fold.
Wen-Xian Li et al.
Luminescence : the journal of biological and chemical luminescence, 26(6), 754-761 (2011-05-14)
A novel ternary complex, TbL(5) L'(ClO(4))(3) · 3H(2)O, two binary complexes, TbL(7) (ClO(4))(3) · 3H(2)O and TbL'(3.5) (ClO(4))(3) · 4H(2)O has been synthesized (using diphenyl sulphoxide as the first ligand L, bipyridine as the second ligand L'). Their composition was
Tomofumi Takuwa et al.
Chemical & pharmaceutical bulletin, 53(5), 476-480 (2005-05-03)
A facile one-pot C-benzylation of various sodium enolates derived from methyl malonate, beta-ketoesters, a beta-cyanoester, a beta-cyanosulfone, ketones and a carboxylic ester is reported. Reaction of alkoxydiphenylsulfonium salts formed by treating various benzyl alcohols with diphenyl sulfide bis(trifluoromethanesulfonate) (derived from
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service