803464
BS(PEG)9 (PEGylated bis(sulfosuccinimidyl)suberate)
Synonym(s):
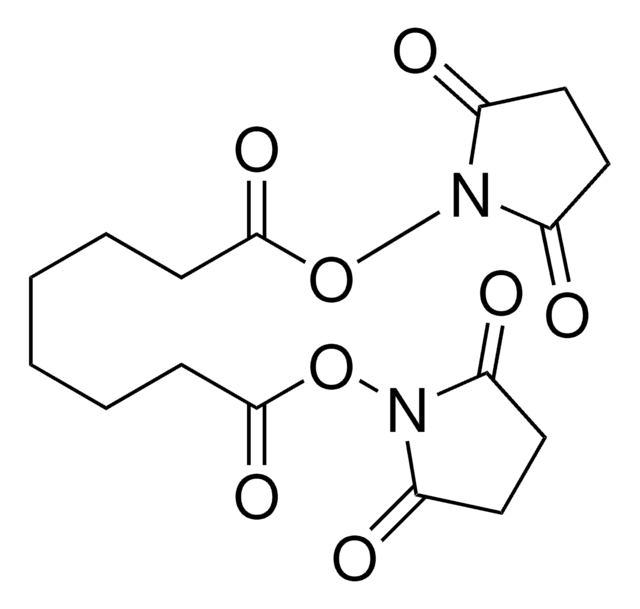
1,31-Bis(2,5-dioxo-1-pyrrolidinyl) 4,7,10,13,16,19,22,25,28-nonaoxahentriacontanedioate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C30H48N2O17
CAS Number:
Molecular Weight:
708.71
UNSPSC Code:
12352200
NACRES:
NA.22
Recommended Products
form
oil
Quality Level
mol wt
708.71
reaction suitability
reagent type: cross-linking reagent
storage condition
desiccated
solubility
water: soluble
shipped in
ambient
storage temp.
−20°C
Related Categories
General description
By contrast to typical PEG reagents that contain heterogeneous mixtures of different PEG chain lengths, this PEG reagent is a homogeneous compound of defined molecular weight and spacer arm length, providing greater precision in optimization and characterization of crosslinking applications.
Application
BS(PEG)9 (PEGylated bis(sulfosuccinimidyl)suberate) can be used as a cross-linker:
- To study the domain structure of BfmR, a regulator of biofilm formation in Acinetobacter baumannii.
- In the study of the mechanism of visual excitation.
Features and Benefits
- Reactive groups: sulfo-NHS ester (both ends)
- Reactive towards: amino groups (primary amines)
- NHS esters react with—NH2 groups at pH 7-9, forming stable amide bonds
- Water-soluble; compare to non-pegylated BS3 (also soluble) and DSS (water-insoluble)
- Membrane-impermeable, allowing for cell surface labeling
- Irreversibly crosslink proteins or peptides by flexible PEG spacer arms
- Polyethylene glycol spacer arms help maintain conjugate solubility
- Pure compound with defined structure and molecular weight, ensuring reproducible protein-modification effects
- PEG spacer provides unique advantages, including increased stability, reduced tendency toward aggregation and reduced immunogenicity
- Ideal for small molecule or peptide conjugations
Caution
This product is sensitive to moisture. The vial is packaged in a resealable bag with a desiccant to reduce exposure to moisture. After cold storage, equilibrate the vial to room temperature before opening to reduce condensation inside the vial. Make fresh solutions. Storage of stock solutions is not recommended. After use, return the vial to the resealable bag. Close the bag and store the product at the recommended temperature.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The structure of the biofilm-controlling response regulator BfmR from Acinetobacter baumannii reveals details of its DNA-binding mechanism
Draughn GL, et al.
Journal of molecular biology, 430(6), 806-821 (2018)
The molecular architecture of photoreceptor phosphodiesterase 6 (PDE6) with activated G protein elucidates the mechanism of visual excitation
Irwin MJ, et al.
Test, 294(51), 19486-19497 (2019)
S Knoller et al.
The Journal of biological chemistry, 266(5), 2795-2804 (1991-02-15)
The superoxide (O2-) forming NADPH oxidase complex of resting phagocytes can be activated in a cell-free system by certain anionic amphiphiles, such as sodium dodecyl sulfate (SDS). For O2- production to occur, the participation of both membrane-associated and cytosol-derived components
G W Cox et al.
Journal of immunology (Baltimore, Md. : 1950), 145(6), 1719-1726 (1990-09-15)
This study was designed to examine the expression and function of IL-2R on murine macrophages. We used a model system of murine macrophage cell lines (ANA-1 and GG2EE) that was established by infecting normal murine bone marrow-derived cells with the
G Mattson et al.
Molecular biology reports, 17(3), 167-183 (1993-04-01)
The various aspects of chemical crosslinking are addressed. Crosslinker reactivity, specificity, spacer arm length and solubility characteristics are detailed. Considerations for choosing one of these crosslinkers for a particular application are given as well as reaction conditions and practical tips
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service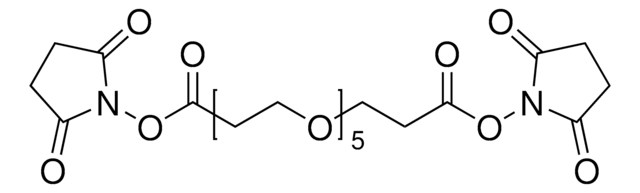




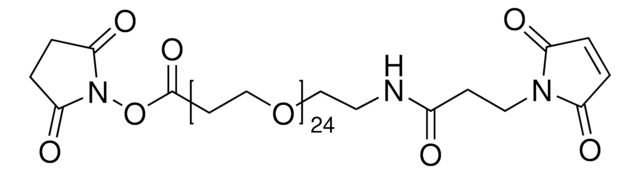
![O,O′-Bis[2-(N-Succinimidyl-succinylamino)ethyl]polyethylene glycol 10,000](/deepweb/assets/sigmaaldrich/product/structures/161/408/20f7ade0-3e2e-4861-ae89-3d81c050059a/640/20f7ade0-3e2e-4861-ae89-3d81c050059a.png)
![O-[N-(3-Maleimidopropionyl)aminoethyl]-O′-[3-(N-succinimidyloxy)-3-oxopropyl]heptacosaethylene glycol ≥90% (oligomer purity)](/deepweb/assets/sigmaaldrich/product/structures/367/864/1c31a65f-501f-4464-8bb3-edff0d131a12/640/1c31a65f-501f-4464-8bb3-edff0d131a12.png)