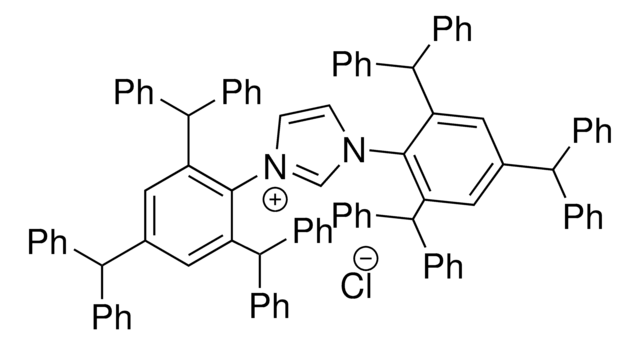
665029
1-(1-Adamantyl)-3-(2,4,6-trimethylphenyl)imidazolinium chloride
Synonym(s):
1-(1-Adamantyl)-3-(2,4,6-trimethylphenyl)-4,5-dihydroimidazolium chloride
About This Item
Recommended Products
form
solid
Quality Level
reaction suitability
reagent type: catalyst
reaction type: Ring-Opening Polymerization
mp
263-280 °C
SMILES string
[Cl-].Cc1cc(C)c(N2CC[N+](=C2)C34CC5CC(CC(C5)C3)C4)c(C)c1
InChI
1S/C22H31N2.ClH/c1-15-6-16(2)21(17(3)7-15)23-4-5-24(14-23)22-11-18-8-19(12-22)10-20(9-18)13-22;/h6-7,14,18-20H,4-5,8-13H2,1-3H3;1H/q+1;/p-1/t18-,19+,20-,22-;
InChI key
MJOMRTQWLPXDJQ-ZFCGJYDVSA-M
Application
- Preparation of chelated ruthenium alkoxybenzylidene imidazolidene carbene pivalate catalysts for Z-selective olefin metathesis
- Use in ring-opening metathesis polymerization and ring-closing metathesis reactions via Grubbs catalyst mediated reactions
- Preparation of adamantyl-substituted N-heterocyclic carbene ligands in second-generation Grubbs-type iridium metathesis catalysis
Legal Information
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Sigma-Aldrich presents an article concerning NHC-based Pd catalysts and ligands for C–C bond formation at sigma-Aldrich.com.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![2-Mesityl-5-methylimidazo[1,5-a]pyridinium chloride 97%](/deepweb/assets/sigmaaldrich/product/structures/495/055/5d86d2cc-b538-4586-9e2c-9e0d870826a7/640/5d86d2cc-b538-4586-9e2c-9e0d870826a7.png)