All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C5H11NO2
CAS Number:
Molecular Weight:
117.15
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Assay
96%
bp
81 °C/20 mmHg (lit.)
mp
34-37 °C (lit.)
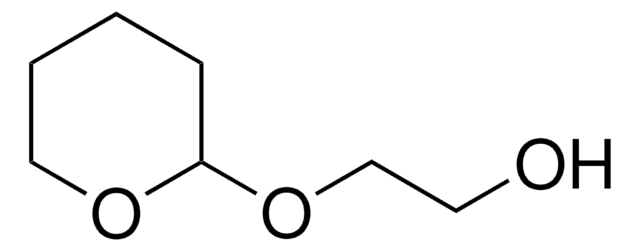
SMILES string
NOC1CCCCO1
InChI
1S/C5H11NO2/c6-8-5-3-1-2-4-7-5/h5H,1-4,6H2
InChI key
NLXXVSKHVGDQAT-UHFFFAOYSA-N
General description
O-(Tetrahydro-2H-pyran-2-yl)hydroxylamine (OTX) is an O-substituted hydroxylamine. The coupling of OTX with alkaline gel electrophoresis has been reported to improve the process of detecting single strand breaks (SSBs) in DNA.
Application
O-(Tetrahydro-2H-pyran-2-yl)hydroxylamine may be used in the synthesis of 2-(5-bromothiophene-2-sulfonamido)-N-(tetrahydro-2H-pyran-2-yloxy)acetamides.
It may be used in the synthesis of the following potential histone deacetylase (HDAC) inhibitors:
It may be used in the synthesis of the following potential histone deacetylase (HDAC) inhibitors:
- 2-[1-(naphthalene-2-sulfonyl)-heterocyclyl]-pyrimidine-5-carboxylic acid (tetrahydropyran-2-yloxy)-amides
- (E)-3-(2-benzyl-1-oxoisoindolin-6-yl)-N-(tetrahydro-2H-pyran-2-yloxy)acrylamide
- 3-(1-benzenesulfonyl-2,3-dihydro-1H-indol-5-yl)-N-hydroxy-acrylamide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
179.6 °F - closed cup
Flash Point(C)
82 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chihiro Shinji et al.
Bioorganic & medicinal chemistry, 14(22), 7625-7651 (2006-08-01)
A series of hydroxamic acid derivatives bearing a cyclic amide/imide group as a linker and/or cap structure, prepared during our structural development studies based on thalidomide, showed class-selective potent histone deacetylase (HDAC)-inhibitory activity. Structure-activity relationship studies indicated that the steric
Patrick Angibaud et al.
European journal of medicinal chemistry, 40(6), 597-606 (2005-06-01)
A series of pyrimidyl-5-hydroxamic acids was prepared for evaluation as inhibitors of histone deacetylase (HDAC). Amino-2-pyrimidinyl can be used as a linker to provide HDAC inhibitors of good enzymatic potency.
Elisa Nuti et al.
European journal of medicinal chemistry, 46(7), 2617-2629 (2011-04-26)
Matrix metalloproteinases (MMPs) are important factors in gliomas since these enzymes facilitate invasion into the surrounding brain and participate in neovascularization. In particular, the gelatinases (MMP-2 and MMP-9), and more recently MMP-25, have been shown to be highly expressed in
Tetrahedron Letters, 45, 133-133 (2004)
Han-Li Huang et al.
PloS one, 7(8), e43645-e43645 (2012-08-29)
Recently, histone deacetylase (HDAC) inhibitors have emerged as a promising class of drugs for treatment of cancers, especially subcutaneous T-cell lymphoma. In this study, we demonstrated that MPT0E028, a novel N-hydroxyacrylamide-derived HDAC inhibitor, inhibited human colorectal cancer HCT116 cell growth
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service