PZ0017
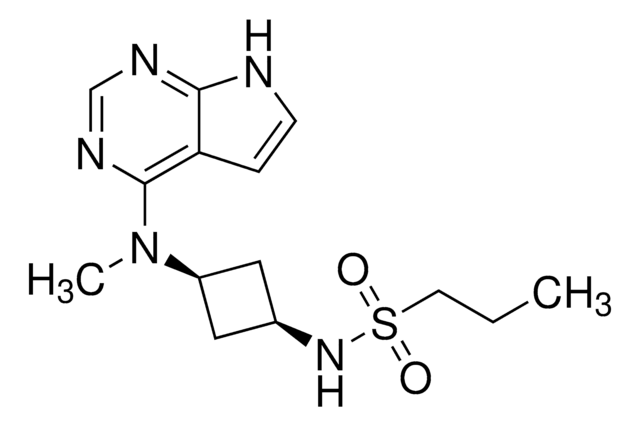
Tofacitinib citrate
≥98% (HPLC), powder, JAK3 inhibitor
Synonym(s):
(3R,4R)-4-Methyl-3-(methyl-7H-pyrrolo[2,3-d]pyrimidin-4-ylamino)-β-oxo-1-piperidinepropanenitrile citrate salt, 3-[(3R,4R)-4-Methyl-3-[methyl-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino]piperidin-1-yl]-3-oxopropanenitrile citrate salt, CP-690550-10, Tasocitinib citrate salt
About This Item
Recommended Products
product name
Tofacitinib citrate, ≥98% (HPLC)
Assay
≥98% (HPLC)
form
powder
color
white to beige
solubility
DMSO: 5 mg/mL (clear solution; warmed)
storage temp.
room temp
SMILES string
OC(=O)CC(O)(CC(O)=O)C(O)=O.C[C@@H]1CCN(C[C@@H]1N(C)c2ncnc3[nH]ccc23)C(=O)CC#N
InChI
1S/C16H20N6O.C6H8O7/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16;7-3(8)1-6(13,5(11)12)2-4(9)10/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20);13H,1-2H2,(H,7,8)(H,9,10)(H,11,12)/t11-,13+;/m1./s1
InChI key
SYIKUFDOYJFGBQ-YLAFAASESA-N
Gene Information
human ... JAK1(3716) , JAK2(3717) , JAK3(3718) , TYK2(7297)
General description
Application
- as a ligand for human serum albumin in fluorescence quenching, dynamic light scattering (DLS) measurements, differential scanning calorimetry and molecular docking studies
- as a medium supplement for full depth articular cartilage (FDC) explants to monitor cytokine-induced proteoglycan loss
- as a Janus kinase inhibitor in MCF7 breast cancer cells
Biochem/physiol Actions
Features and Benefits
Other Notes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Repr. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Related Content
Discover Bioactive Small Molecules for Kinase Phosphatase Biology
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service