All Photos(1)
About This Item
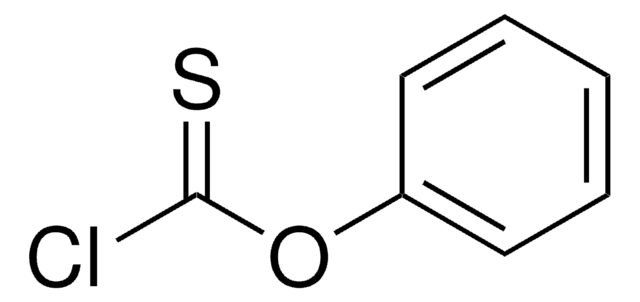
Linear Formula:
ClCS2C6H5
CAS Number:
Molecular Weight:
188.70
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
refractive index
n20/D 1.6688 (lit.)
bp
135 °C/15 mmHg (lit.)
density
1.331 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
ClC(=S)Sc1ccccc1
InChI
1S/C7H5ClS2/c8-7(9)10-6-4-2-1-3-5-6/h1-5H
InChI key
XEXIHCWFFNGQDE-UHFFFAOYSA-N
General description
Kinetics and mechanism of solvolysis reaction of phenyl chlorodithioformate was reported.
Application
Phenyl chlorodithioformate was used in synthesis of phenyl arylsulfonyl-alkyl-dithiocarbamates. It was also used in synthesis of S-phenyl O-(4-nitrophenyl)dithiocarbonate.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of Phenyl Arylsulfonyl-alkyl-dithiocarbamates and Their Hydrolytic Reactivity in Hydroxide and Hydroperoxide Media.
Norberto F, et al.
European Journal of Organic Chemistry, 2005(21), 4710-4714 (2005)
Kinetics and mechanism of the reactions of aryl chlorodithioformates with pyridines and secondary alicyclic amines.
Castro EA, et al.
Journal of Physical Organic Chemistry, 22(11), 1030-1037 (2009)
Myung Gil Choi et al.
The Analyst, 144(24), 7263-7269 (2019-11-07)
A novel hypochlorous-acid-selective signaling probe based on the carbonodithioate derivative of resorufin (RT-1) was developed. Probe RT-1 showed prominent colorimetric and turn-on type fluorescence signaling behavior exclusively toward hypochlorous acid, induced by oxidative hydrolysis, to regenerate resorufin dye. Hypochlorous acid
The nucleofuge in the pyridinolysis of O-(4-nitrophenyl) S-aryl thio and dithiocarbonates.
Castro EA, et al.
Journal of Physical Organic Chemistry, 25(11), 994-994 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service