Batteries, Supercapacitors & Fuel Cells
Batteries, fuel cells, and supercapacitors are systems using different electrochemical energy storage and conversion mechanisms but similar electrochemical features for high energy and high-power density applications.
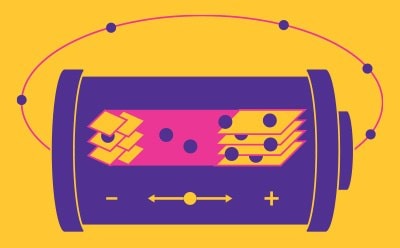
Batteries
A battery consists of electrodes (cathode (+) and anode (-)), a conductive electrolyte, and a separator between the anode and the cathode. In rechargeable lithium-ion batteries (LIB), monovalent lithium cations migrate between the electrodes. When discharging, the anode (-) oxidizes (loses electrons) and the cathode undergoes reduction (gain of electrons). Upon charging, this process is reversed. Due to their high energy, power density, improved safety and lower material costs, LIBs have revolutionized the electronics industry and are integrated in many aspects of our lives, from mobile devices to electric vehicles. In 2019, the Nobel Prize in Chemistry was awarded to the scientists who developed the LIB technology.
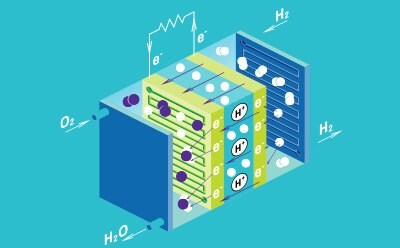
Fuel Cells
Fuel cells consist of an anode, cathode, and a conductive electrolyte, and are often connected in a series to form a stack to increase the total amount of generated electricity. The electrode is comprised of a porous material that is coated with a catalyst to generate electricity. There are five main types of fuel cell types, which are differentiated by the type of electrolyte used: polymer electrolyte membrane, solid oxide, phosphoric acid, alkaline, and molten carbonate. Polymer electrolyte membrane, also known as proton-exchange membrane, (PEM) technology is considered the most promising to replace alkaline fuel-cell technology.
Fuel cells have been developed as an alternative energy technology, due to their high efficiencies, low emissions, and low environmental impact, outcompeting traditional combustion engines. Fuel cells generate only heat and water as waste products, making them a promising candidate for future power sources in a wide variety of applications, including portable devices, stationary devices, and transportation solutions.
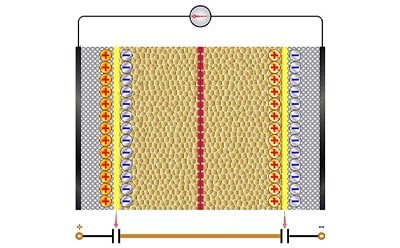
Supercapacitors
The components of supercapacitors are similar to batteries. However, supercapacitors are characterized by their charge storage capabilities. The electrode materials contribute to the storage performance of a supercapacitor and can be divided into three categories: double layer capacitors that act electrostatically, pseudo-capacitors that act electrochemically, and hybrid capacitors that utilize both.
Supercapacitors are a high-density energy source with high energy storage capacity, long shelf life, and quick charging capabilities making them ideal for applications in hybrid vehicles, portable devices, and energy harvesting.
Related Technical Articles
Related Protocols
- Surfactant-assisted dispersion of single-walled carbon nanotubes for debundling or exfoliation in dispersion procedures.
- See All (1)
Find More Articles and Protocols
To continue reading please sign in or create an account.
Don't Have An Account?