B3561
BAY 73-6691
≥98% (HPLC), powder
Sinónimos:
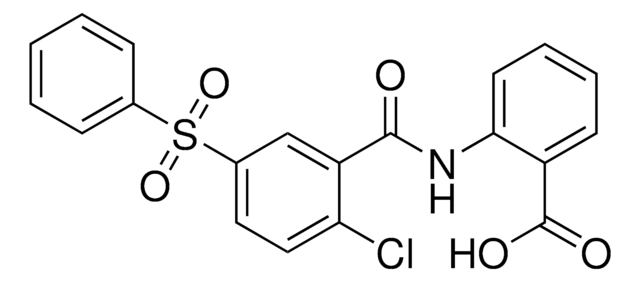
1-(2-Chlorophenyl)-6-[(2R)-3,3,3-trifluoro-2-methylpropyl]-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidine-4-one
About This Item
Productos recomendados
Quality Level
assay
≥98% (HPLC)
form
powder
color
off-white
solubility
DMSO: >20 mg/mL
originator
Bayer
storage temp.
2-8°C
SMILES string
C[C@H](CC1=Nc2c(cnn2-c3ccccc3Cl)C(=O)N1)C(F)(F)F
InChI
1S/C15H12ClF3N4O/c1-8(15(17,18)19)6-12-21-13-9(14(24)22-12)7-20-23(13)11-5-3-2-4-10(11)16/h2-5,7-8H,6H2,1H3,(H,21,22,24)/t8-/m1/s1
InChI key
FFPXPXOAFQCNBS-MRVPVSSYSA-N
Application
Biochem/physiol Actions
Features and Benefits
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 2 Oral - Aquatic Chronic 4 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
Cyclic nucleotide phosphodiesterases (PDEs) catalyze the hydrolysis of cAMP and/or cGMP. There are 11 different mammalian PDE families.
Contenido relacionado
Cyclic nucleotides, including cyclic AMP (cAMP), cyclic GMP (cGMP) and cyclic ADP-ribose, have been extensively studied as second messengers of intracellular events initiated by activation of GPCRs. cAMP modifies cell function in all eukaryotic cells, principally through the activation of cAMP-dependent protein kinase (PKA), but also through cAMP-gated ion channels and guanine nucleotide exchange factors directly activated by cAMP.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico