M-145
Milnacipran hydrochloride solution
1.0 mg/mL in methanol (as free base), ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Productos recomendados
grade
certified reference material
form
liquid
feature
Snap-N-Spike®/Snap-N-Shoot®
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
concentration
1.0 mg/mL in methanol (as free base)
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
clinical testing
format
single component solution
storage temp.
−20°C
SMILES string
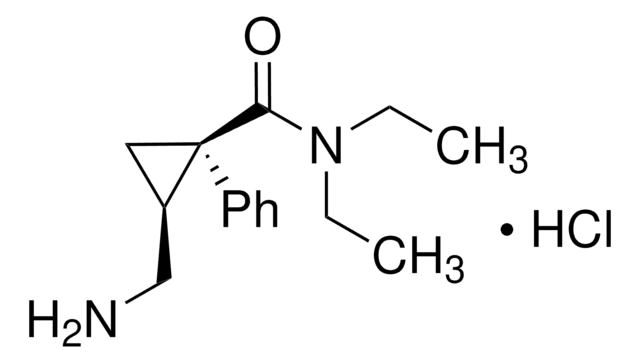
CCN(CC)C([C@]1(C2=CC=CC=C2)C[C@@H]1CN)=O.Cl
InChI
1S/C15H22N2O.ClH/c1-3-17(4-2)14(18)15(10-13(15)11-16)12-8-6-5-7-9-12;/h5-9,13H,3-4,10-11,16H2,1-2H3;1H/t13-,15+;/m1./s1
InChI key
XNCDYJFPRPDERF-PBCQUBLHSA-N
General description
Application
- Anesthetic and Analgesic Applications of Milnacipran: Review on new serotonin-norepinephrine reuptake inhibitors, highlighting Milnacipran hydrochloride′s role in anesthetic and analgesic applications, discussing its impact on pain management strategies (Fanelli et al., 2021).
- Sustained Release Delivery System for Milnacipran: Development of a novel chitosan-polycaprolactone based mucoadhesive gastro-retentive drug delivery system for Milnacipran HCl, aimed at improving bioavailability and enhancing patient compliance (Hussain et al., 2020).
- Comparative Review of Levomilnacipran and Milnacipran: Analysis of Levomilnacipran′s effectiveness compared to Milnacipran in treating major depressive disorder, focusing on efficacy, tolerability, and patient outcomes (Gautam et al., 2019).
- Antidepressant Efficacy and Side Effects: Network meta-analysis of 21 antidepressants, including Milnacipran, assessing their side effect profiles and comparative tolerability in the acute treatment of major depression (Tomlinson et al., 2019).
Legal Information
signalword
Danger
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
target_organs
Eyes
Storage Class
3 - Flammable liquids
wgk_germany
WGK 1
flash_point_f
49.5 °F - closed cup
flash_point_c
9.7 °C - closed cup
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico