ADE000689
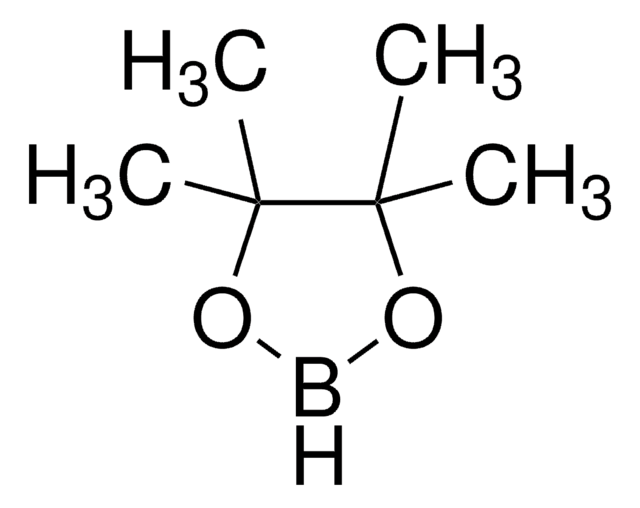
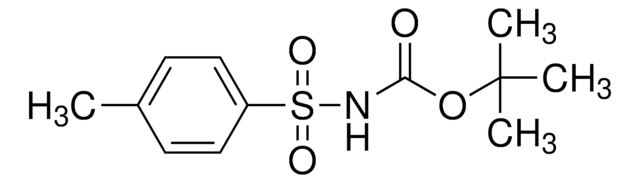
tert-Butyl 3-((3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin-2-yloxy)methyl)pyrrolidine-1-carboxylate
AldrichCPR
About This Item
Recommended Products
form
solid
SMILES string
CC(C)(C)OC(=O)N1CCC(COc2ncccc2B3OC(C)(C)C(C)(C)O3)C1
InChI
1S/C21H33BN2O5/c1-19(2,3)27-18(25)24-12-10-15(13-24)14-26-17-16(9-8-11-23-17)22-28-20(4,5)21(6,7)29-22/h8-9,11,15H,10,12-14H2,1-7H3
InChI key
YDXOOIAHGJQCCR-UHFFFAOYSA-N
Other Notes
NOTWITHSTANDING ANY CONTRARY PROVISION CONTAINED IN SIGMA-ALDRICH′S STANDARD TERMS AND CONDITIONS OF SALE OR AN AGREEMENT BETWEEN SIGMA-ALDRICH AND BUYER, SIGMA-ALDRICH SELLS THIS PRODUCT "AS-IS" AND MAKES NO REPRESENTATION OR WARRANTY WHATSOEVER WITH RESPECT TO THIS PRODUCT, INCLUDING ANY (A) WARRANTY OF MERCHANTABILITY; (B) WARRANTY OF FITNESS FOR A PARTICULAR PURPOSE; OR (C) WARRANTY AGAINST INFRINGEMENT OF INTELLECTUAL PROPERTY RIGHTS OF A THIRD PARTY; WHETHER ARISING BY LAW, COURSE OF DEALING, COURSE OF PERFORMANCE, USAGE OF TRADE OR OTHERWISE.
Legal Information
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
The synthesis of biaryl compounds via the Suzuki–Miyaura coupling reaction has become more commonplace now that many arylboronic acids are readily available.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)