Superoxide Dismutase
Superoxide Dismutase
Superoxide Dismutase (SOD) catalyzes the reduction of superoxide anions to hydrogen peroxide. It plays a critical role in the defense of cells against the toxic effects of oxygen radicals. SOD competes with nitric oxide (NO) for superoxide anion, which inactivates NO to form peroxynitrite. Therefore, by scavenging superoxide anions, SOD promotes the activity of NO. SOD has suppressed apoptosis in cultured rat ovarian follicles, neural apoptosis in neural cell lines, and transgenic mice by preventing the conversion of NO to peroxynitrate, an inducer of apoptosis.8,9,10 Covalent conjugation of superoxide dismutase with polyethylene glycol (PEG) has been found to increase the circulatory half-life and provides prolonged protection from partially reduced oxygen species. 7
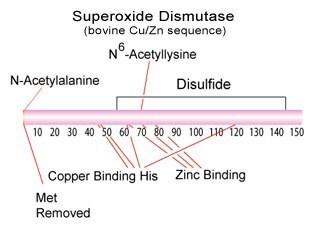
Figure 1.Superoxide Dismutase Structure
Superoxide Dismutase Products
| Superoxide Dismutase Enzymes Superoxide Dismutase Substrates Superoxide Dismutase Inhibitors |
Superoxide Dismutase Generators Superoxide Dismutase Indicators Superoxide Dismutase Detection |
Superoxide Dismutase Properties
Enzyme Commission (EC) Number: 1.15.1.1 ( BRENDA | IUBMB )
- The bovine enzyme exists as a dimeric copper-zinc containing protein with a molecular weight of 2 X 16,300.3
- The E. coli enzyme exists as a dimeric manganese or iron containing glycoprotein with a molecular weight of 2 x 22,000.4,5
- The human enzyme exists as a tetrameric copper-zinc or manganese containing glycoprotein with a molecular weight of 2 X 23-28,300.3,6
Superoxide Dismutase Reaction

Figure 2.SOD Reaction
Km for O2- for bovine SOD = 0.35 mM
Superoxide Dismutase Enzyme Assay
Superoxide Dismutase activity is measured as the inhibition of the rate of reduction of Cytochrome c by the superoxide radical, observed at 550 nm:
Cytochrome c (oxidized) +O2-. → Cytochrome c (reduced) + O2
The superoxide radical is produced enzymatically by the reaction with xanthine oxidase:
Xanthine + O2 + H2O  Uric acid + O2-. + H+
Uric acid + O2-. + H+
SOD Assay Procedure
Cell Damage
Cell damage is induced by reactive oxygen species (ROS). ROS are either free radicals, reactive anions containing oxygen atoms, or molecules containing oxygen atoms that can either produce free radicals or are chemically activated by them. Examples are hydroxyl radical, superoxide, hydrogen peroxide, and peroxynitrite. The main source of ROS in vivo is aerobic respiration, although ROS are also produced by peroxisomal β-oxidation of fatty acids, microsomal cytochrome P450 metabolism of xenobiotic compounds, stimulation of phagocytosis by pathogens or lipopolysaccharides, arginine metabolism, and tissue specific enzymes.1,2
Under normal conditions, ROS are cleared from the cell by the action of superoxide dismutase (SOD), catalase, or glutathione (GSH) peroxidase. The main damage to cells results from the ROS-induced alteration of macromolecules such as polyunsaturated fatty acids in membrane lipids, essential proteins, and DNA. Additionally, oxidative stress and ROS have been implicated in disease states, such as Alzheimer's disease, Parkinson’s disease, cancer, and aging.
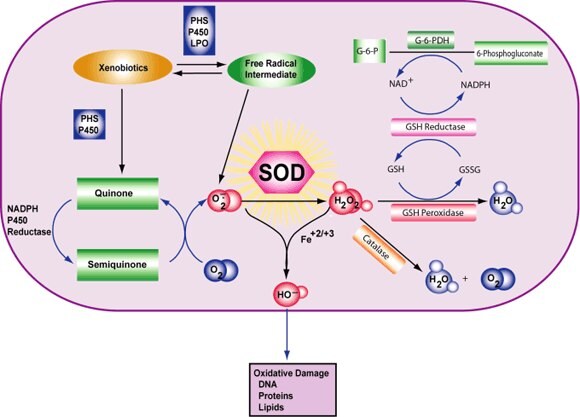
Figure 4.Superoxide Dismutase Reaction
References
若要繼續閱讀,請登入或建立帳戶。
還沒有帳戶?