Solubility Rules | Solubility of Common Ionic Compounds
Solubility Chemistry
It’s important to know how chemicals will interact with one another in aqueous solutions. Some compounds or solutes will dissolve, others will yield a precipitate or solid, and a few react with water.
You’ve probably run into solubility questions in your everyday life. Hot chocolate or flavored dry beverage mixes that don’t evenly dissolve in water would be examples of unwanted precipitates. Limescale or soap scum are precipitates left behind when water with higher mineral content evaporates and introduces previously dissolved metal cations to carbonates or soap anions.
Solubility is applicable to many laboratory processes and is also important in medicine. Some ions can be toxic when they separate in a solution but are helpful as part of a compound.
A saturated solution is one in which the maximum amount of solute has been dissolved. The opposite is a dilute solution; this solution can accept more solute.
Pressure and temperature affect solubility. This page discusses the solubility of compounds in water at room temperature and standard pressure. A compound that is soluble in water forms an aqueous solution.
Solubility Rules
Which ions are soluble? |
|---|
Which ions are slightly soluble? |
|---|
Which ions are insoluble? |
|---|
How to Use Solubility Rules
Identify the compound whose solubility you want to check. It can be helpful to write out the empirical formula so you can identify the ions that make up the compound.
Look up each ion in the solubility rules. Check the left-hand column for the general rule, and look in the right-hand column to make sure you noted any exceptions.
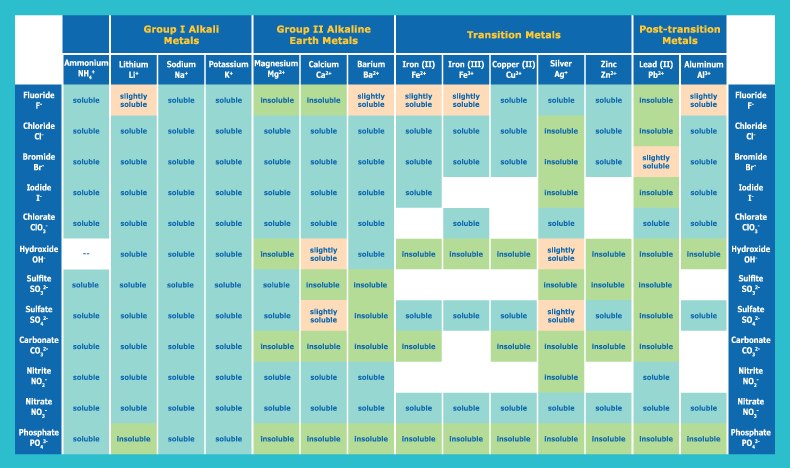
Alternatively, you can look up ions in the solubility chart. You might find this easier. Cations are listed across the top, and anions are listed vertically. Find the cell where your cation column and ion row meet to determine solubility of the resulting compound.
Our solubility rules are not exhaustive. You may need to reference a periodic table if you’re looking up less common compounds.
Solubility Rules Chart
Solubility Chart Download for Study and Classroom Use
Our basic solubility chart is available free for download. Feel free to share it with friends, students, or teachers. Just make sure to link back to this page.
Want to share some feedback about our study tools? Use the links above to contact us or leave a comment on one of our social media accounts.
Solubility Definitions
When discussing solubility, it’s useful to follow agreed standard definitions. The resources above present some general rules and loose definitions.
The United States Pharmacopeia (USP), a nonprofit organization committed to establishing standards for medicines, food ingredients, dietary supplement products, and ingredients, has established the definitions below. These definitions are used by other major pharmacopeia organizations throughout the world and are often paired with exact measurements for more precise application needs.
若要繼續閱讀,請登入或建立帳戶。
還沒有帳戶?