Autophagy Assays
Introduction
Autophagy is a highly regulated homeostatic degradative process where cells destroy their own components via the lysosomal machinery and recycle them. This process is associated with diverse diseases including Alzheimer’s disease1, aging2, cancer3 and Crohn’s disease. Via extensive crosstalk with pro-apoptotic signaling pathways, autophagy can also contribute to cell death and greatly influence general cell health. Malfunctions of autophagy can influence longevity and productivity of cells to function at full capacity. Elucidating the correlation between autophagy and apoptotic cell death has become the focus of a great deal of research, particularly in tumor biology. On one hand, autophagy may induce cell death by degrading essential components; on the other hand, it may facilitate survival of cancer cells under unfavorable metabolic conditions4.
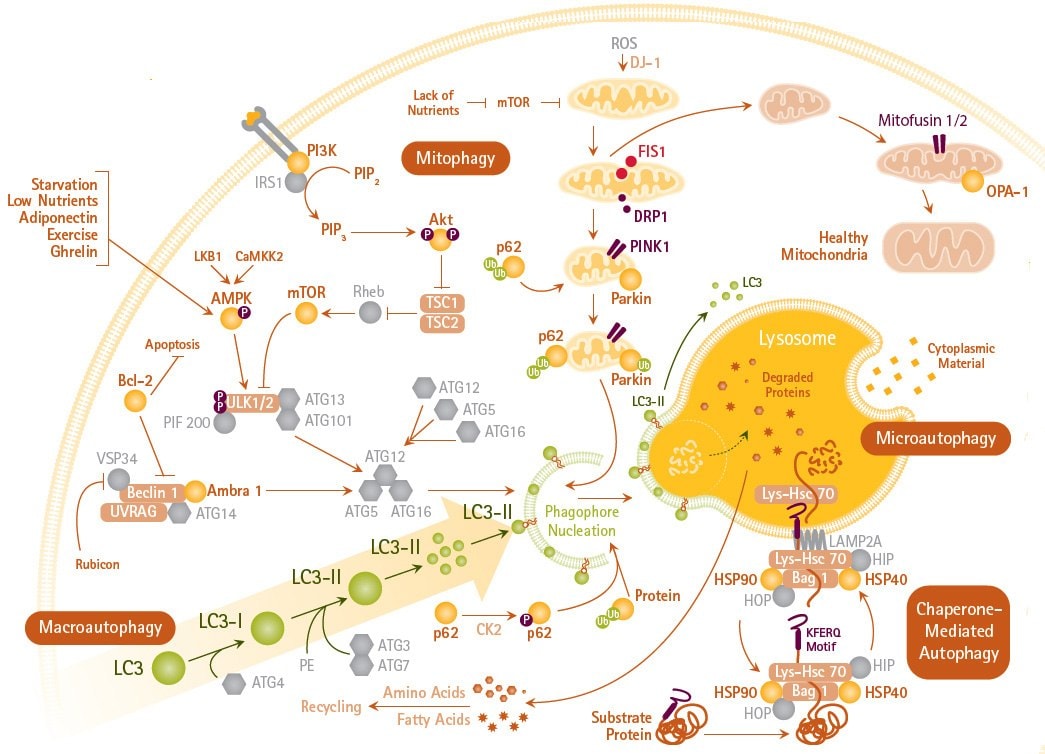
Figure 1.Autophagy Mechanisms and Signaling Pathways. Autophagy (including macroautophagy, mitophagy and microautophagy) is an intracellular degradation system that delivers cytoplasmic constituents to the lysosome often mediated by LC3.
Live Cell LC3 Lentiviral Fluorescent Biosensors
Members of the LC3 family play a key role in the maturation of the autophagosome, the central organelle of autophagy5. LC3 precursors, diffusely distributed in the cytosol, are proteolytically processed to form LC3-I. Upon initiation of autophagy, the C-terminal glycine is modified by addition of a phosphatidylethanolamine to form LC3-II, which translocates rapidly to nascent autophagosomes in a punctate distribution.
LentiBrite™ LC3 lentiviral particles provide bright GFP/RFP fluorescence and precise localization to enable live cell analysis of autophagy in difficult-to-transfect cell types. Analyze Live Cell Autophagy in cell cultures using pre-packaged high-titer fluorescent biosensors.
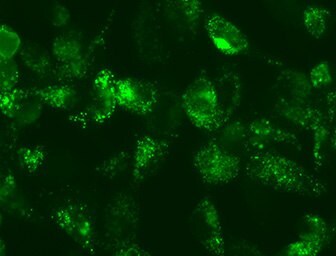
Figure 2.Live Cell LC3 Lentiviral Fluorescent Biosensors
LC3-II Autophagy Enrichment Kits
LC3 precursors are proteolytically processed to form LC3-I, which is diffusely distributed in the cytosol. Upon initiation of autophagy, the C-terminal glycine of LC3-I is modified by addition of a phosphatidylethanolamine (PE) to form LC3-II, which translocates rapidly to nascent autophagosomes in a punctate distribution5. However, detecting and interpreting the relative amounts of LC3-I and LC3-II in standard assay methods can be complicated.
The LC3-II Enrichment Kit enables sensitive and accurate quantification of autophagosome density by utilizing a selective permeabilization procedure that removes cytosolic LC3-I and retains autophagosome-bound LC3-II. This procedure reveals quantities of LC3-II, without interference from LC3-I, by Western blotting analysis or Flow Cytometry applications.
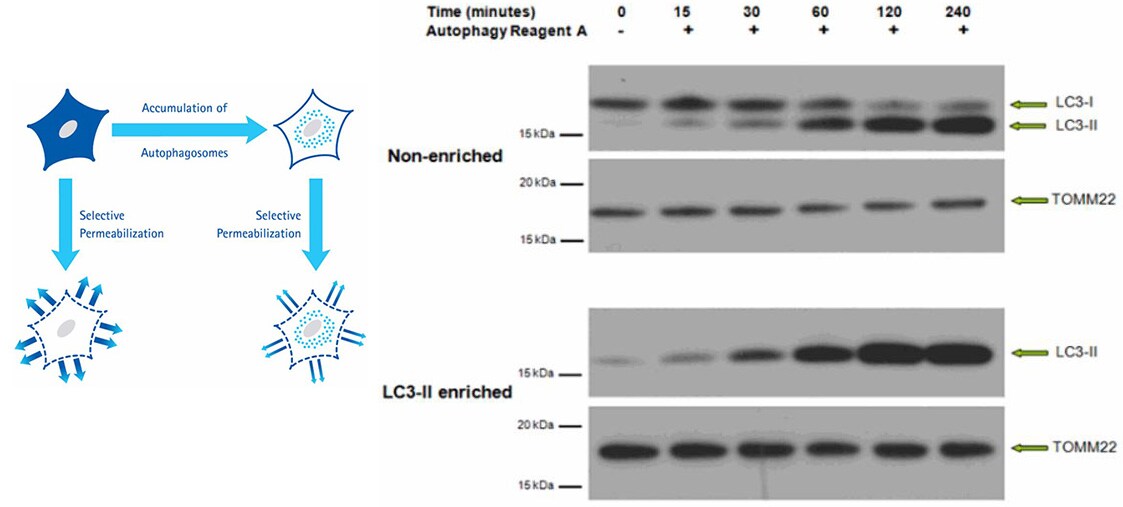
Figure 3.Western Blot of non-enriched lysate and LC3-II-enriched protein fraction from HeLa cells prepared with the LC3-II Enrichment Kit. Proteins were subjected to SDS-PAGE and were transferred to a PVDF membrane. The membrane was probed with primary anti-LC3 and anti-TOMM22, followed by secondary antibodies. The blot was developed by enhanced chemiluminescence. Immunoblotting results of non-enriched lysates indicate the LC3-I signal decreases over time after induced autophagy, as the LC3-II signal increases. After enrichment, the LC3-I signal is no longer detectable and the LC3-II signal is retained
Flow Cytometry Autophagy Detection
FlowCellect™ GFP-LC3 Reporter Autophagy Assay Kits provide a quantitative solution for studying autophagy and measuring potency of autophagy inducers using flow cytometry. This kit has four unique features:
- The use of a proprietary selective permeabilization solution discriminates between cytosolic LC3 from autophagic LC3 by extracting the soluble cytosolic proteins, while protecting LC3 which has been sequestered into the autophagosome;
- The use of a monomeric GFP is used as a reporter to facilitate the translocation of the fusion protein, as other forms of GFP form dimers and aggregate when over-expressed in the cells, thus making it difficult to be extracted from cytoplasm and impossible to measure translocation by flow cytometry;
- Since autophagy is a constitutive cellular degradation process, the use of an autophagy detection reagent will prevent the lysosomal degradation of LC3, allowing its quantification by flow cytometry.
- The monomeric GFP used in our fusion protein to LC3 is attached on the 5' end (N-terminal fusion), protecting the GFP from Atg4 cleavage, thus, allowing its visualization within the autophagosomes.
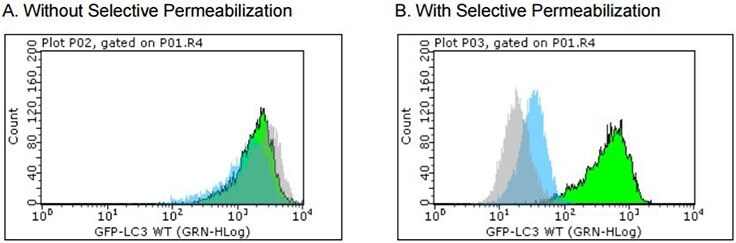
Figure 4. GFP-LC3 stable reporter cell line for detecting the rate of autophagy and for drug screening.In (A), without Selective Permeabilization, no shift of GFP-LC3 level is detected using flow cytometry before and after starvation (induction of autophagy). The position of the histograms indicates the high level of GFPLC3 expression in the cytoplasm. In (B), with Selective Permeabilization, the GFP-LC3 level remains high in autophagosomes when starved in the presence of lysosome inhibitor (green); even without the inhibitor, a slight shift is observed when starved (blue). All the cytosolic GFP-LC3 is washed away if no autophagy is induced by starvation (gray).
References
若要繼續閱讀,請登入或建立帳戶。
還沒有帳戶?