Extractables Study with LC-UV/MS - Efficient identification and quantification of unknown extractables of a single-use system using the certified reference material (CRM) mix for extractables and leachables
Tim Mueller, Scientist Liquid Chromatography-Mass Spectrometry, Marc Gemeinder, Project Manager for Extractables and Leachables Studies, Saskia Haehn, Manager Extractables and Leachables Laboratory, Matthias Nold, Product Manager Reference Materials
Merck
In issue 8 of the Analytix Reporter magazine, we presented a GC/MS method to detect extractables and leachables (E&L) in a single-use equipment. In addition, we also demonstrated the convenience of using a TraceCERT® extractables and leachables certified reference material (CRM) mix for GC to efficiently detect and quantify the most common extractables. Here we present the use of a corresponding CRM mix for liquid chromatography methods by means of an LC-UV/MS based E&L study.
Single-use systems (SUS) made of polymers are commonly used components in the manufacturing or handling of drugs. This direct contact can lead to the contamination of the drug by leaching of the polymeric material components into the product.
To manage this risk, it is crucial to understand the compounds that might potentially migrate from a material (extractable study) and also the quantities at which such a migration is occurring under certain conditions (leachable study).
Leachables: Chemical compounds that migrate into a drug formulation from any product contact material (e.g., single-use systems) because of direct contact under a typical process or storage conditions; Leachables may affect the toxicity or efficiency of the drug product
Extractables: Chemical compounds that are extracted from any product contact material usually under extreme conditions (harsh solvents, exaggerated time, and temperature); an Extractables profile represents a worst-case Leachables profile
Single-use Systems (SUS): Usually polymeric, disposable equipment for bioprocessing used in the manufacturing of pharmaceuticals
- Advantages: Flexibility, no need for cleaning validation, low investment, no cross contamination
- Examples of SUS: Bioreactors, disposable filters, or tubing
As described in the BPOG (BioPhorum Operations Group) guidelines1 and USP <665> guidelines for polymeric components and systems2 (draft version), investigations regarding extractables should be performed using various solvents and incubation times with the analysis done using a variety of analytical methods applied to the extracts.
A well-suited method to analyze non-volatile extractables such as additives, impurities, polymer components, or degradation products is liquid chromatography-ultraviolet spectroscopy/mass spectrometry (LC-UV/MS). UV and MS are chosen to detect a wide range of extractables. As generally the exact composition of the polymeric material is unknown, a non-targeted analysis is required that involves the detection and identification of any potential extractable.
To facilitate this type of analysis, we have developed a CRM mixture for 21 extractables typically found in LC-UV/MS studies. This CRM mix is not only helpful for a quick identification of unknown extractables but can also be used for quantification with traceability to a NIST SRM. Since the mix contains a wide variety of substance classes, it is also suitable to check the analytical method to reduce the risk of overlooking potential extractables. The 21 compounds in the mix are listed in Table 2. The corresponding single component reference materials are shown under “Related Products” below.
In the following, an application is described using the Extractables and Leachables Screening Standard for LC mix, for the identification and quantification of the main extractables within an extractable study of a filter.
LC Method for Extractables Testing
The applied instrument parameters for an extractable study on single-use equipment (filter) are summarized in Table 1. According to the BPOG protocol1, the separation was performed on a C18 column (Ascentis® C18 Column: 15 cm x 2.1 mm, 3 µm). A representative sample was taken after 24 hours of extraction at 40 °C under orbital rotation with 50% ethanol. The sample and the standard mix were run in one sequence.
Results & Discussion
The chromatograms of the Extractables and Leachables Screening Standard for LC are shown in Figure 1-3 (UV, ESI pos, ESI neg, peak IDs in Table 2). All 21 reference compounds were detected by the combination of UV-MS detector with almost complete separation. Sixteen reference compounds could be detected with UV (220 nm), 13 reference compounds with ESI positive, and 14 reference compounds with ESI negative ionization. By matching of retention time and m/z ratio, Pentaerythritol tetrakis(3,5-di-tert- butyl-4-hydroxyhydrocinnamate) (Irganox 1010) was identified as the main extractable during the extraction of the single-use filter (Figure 4). A quantitative analysis against the Pentaerythritol tetrakis(3,5-di- tert-butyl-4-hydroxyhydrocinnamate) (Irganox 1010) peak within the Extractables and Leachables Screening Standard for LC could be performed based on UV (220 nm), ESI positive or ESI negative chromatograms.

Figure 1.Extractables and Leachables Screening Standard for LC, UV (220nm), 10 mg/L in acetonitrile.

Figure 2.Extractables and Leachables Screening Standard for LC, ESI positive, 10 mg/L in acetonitrile.

Figure 3.Extractables and Leachables Screening Standard for LC, ESI negative, 10 mg/L in acetonitrile.
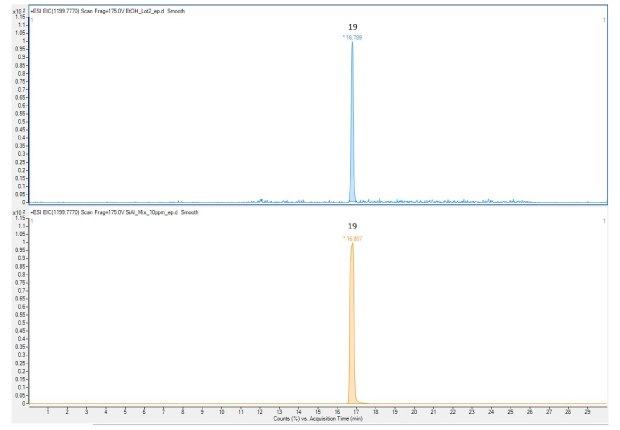
Figure 4.Representative sample of a single-use equipment extraction. Top: 50% EtOH Sample, bottom: Extractables and Leachables Screening Standard for LC
Conclusion
The example shown demonstrates the applicability and value of the Extractables and Leachables Screening Standard in LC analysis for a reliable analysis of the most common extractables resulting from single-use equipment. The shown LC-UV/MS method using an Ascentis® C18 column provided a reliable identification and quantification of the 21 components in the mix.
References
若要繼續閱讀,請登入或建立帳戶。
還沒有帳戶?