Desalting Samples Using Mini Dialysis Kit
Mini Dialysis Kit is designed for the dialysis of small sample volumes with minimal handling and sample loss, offering a simple solution to the handling problems of low-volume dialysis and reducing the pronounced streaking on 2-D gels caused by low-molecular-weight contaminants (Figure 1). The kit contains dialysis tubes, each of which consists of a sample tube with a cap that is fitted with a dialysis membrane. Sample is easily and quantitatively transferred into and out of the tube by pipetting.

Figure 1.Effect of dialysis on 2-D resolution. Sample: E. coli protein extracted with 15 mM NaCl, 8 M urea, 0.5% Pharmalyte pH 3–10, 2% CHAPS. Dialysis: Mini Dialysis Kit 8 kDa, 250 µl, 17 h against 8 M urea. First dimension: Approximately 400 µg E. coli protein, 13-cm Immobiline DryStrip pH 3–10 NL, Ettan IPGphor Isoelectric Focusing System 32 kVh. Second dimension: SDS-PAGE (12.5%), SE 600 vertical electrophoresis system (16 × 16 cm gel). Stain: Colloidal Coomassie™ G-250.
The capped tube is inverted in a stirred beaker containing the solution against which the sample is to be dialyzed. Salts and molecules smaller than the molecular weight cut-off of the dialysis membrane are effectively exchanged through the membrane. Following dialysis, the tube is centrifuged briefly. This forces the entire contents of the dialysis tube into the bottom of the tube, ensuring essentially 100% recovery. The dialyzing cap is replaced with a normal cap for storage of the dialyzed sample (Figure 2). The kit is available with a choice of molecular weight cut-off (either 1 kDa or 8 kDa) and a choice of tubes for sample volumes of either 250 µL or 2 ml. Each kit contains dialysis tubes and associated accessories sufficient for preparing 50 samples.
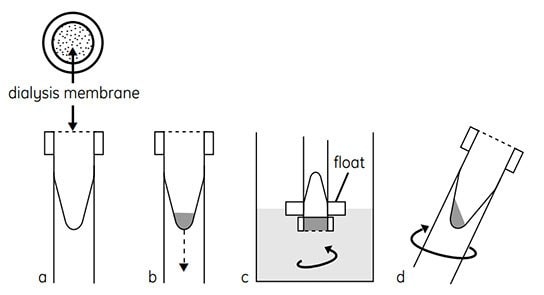
Figure 2.Schematic of the method used in Mini Dialysis Kit. (a) Cap with dialysis membrane, conical inner sample tube. (b) Introduce sample, screw on cap, and slide tube into float. (c) Invert and dialyze while stirring. (d) Spin briefly to collect sample.
Dialysis times of a few hours to overnight are sufficient to reduce ionic contaminants to a level that does not interfere with first-dimension IEF separation.
Since some detergents, notably Triton X-100 and SDS, form high-molecular-weight micelles at low concentration, they cannot be effectively removed by dialysis. Other techniques, such as sample precipitation with 2-D Clean-Up Kit, must be used to remove these detergents.
Protocol: Mini Dialysis Kit
Components supplied
Dialysis tubes with caps incorporating a dialysis membrane (tubes for up to 250 µL or 2 mL sample are included in the kit), caps (standard tube caps to seal the tubes following dialysis), floats (floating plastic sponges to suspend the inverted dialysis tubes in the dialysis solution).
Required but not provided
Centrifuge (dependent on size of dialysis tube) capable of low speeds.
Preliminary notes
Prior to dialysis, samples for native IEF should be solubilized in water while samples for denaturing IEF for 2-D work should be solubilized in a solution containing urea, reductant, and nonionic detergent. See sections 1.6.1 and 1.6.2 for details.
Handle dialysis tubes and caps with gloves.
Dialysis tubes are supplied in 0.05% (w/v) sodium azide and require rinsing before use.
- Rinse dialysis tube and cap with distilled or deionized water (Figure 2A). Keep cap covered with water until needed. Do not allow cap with dialysis membrane to dry out.
- Remove cap from water. Remove excess water with a micropipette. Ensure that the cap is tightly sealed.
- Add sample to dialysis tube and replace dialysis cap (Figure 2B). For 250 µL dialysis tubes, use 10–250 µL of sample. For 2-mL dialysis tubes, use 200 µl-2 mL of sample.
- Invert dialysis tube ensuring that entire sample rests on dialysis membrane. If the sample is viscous and does not initially rest on the membrane, the dialysis tube can be centrifuged in the inverted position at 10–100 × g for no more than 6 s.
Spinning longer or faster may rupture the membrane.
- Secure dialysis tube to one of the floats provided. Place dialysis tube and float assembly (cap-end down) in a beaker of the solution to be dialyzed against (e.g. water or 1% glycine for native IEF or sample buffer containing urea, reducing agent, and nonionic detergent for denaturing IEF; see sections 1.6.1 and 1.6.2 for details). Check that the dialysis membrane fully contacts the dialysis solution and that no large air bubbles are trapped beneath the dialysis membrane. Remove any air bubbles by tilting the tube or squirting dialysis solution onto the membrane.
See section below on dialysis solutions.
- Dialyze while stirring (Figure 2C). During dialysis, invert dialysis tube to thoroughly mix contents. Note: Optimal dialysis time depends on several factors, including the nature and volume of the sample, the molecular weight cut-off of the dialysis membrane and the temperature. Normally, dialysis for 2 h to overnight is sufficient to reduce ionic contaminants to a level that does not interfere with IEF separation. Dialysis may be carried out at 4–8 °C to minimize sample degradation or modification, but this will slow down the dialysis. Dialysis can be conducted at room temperature if degradation or modification is not a concern.
- After dialysis, centrifuge dialysis tube for 6 s at 500–1000 × g to collect sample (Figure 2D).
- Remove dialysis cap and replace with normal cap for storage. The protein concentration of the sample is best determined using the 2-D Quant Kit. The kit allows accurate quantitation of protein in the presence of detergents, reductants, and chaotropes that are incompatible with other assays.
Dialysis solution
A substantial reduction in interfering ions can be achieved by dialyzing 2-D samples against a solution volume at least 40 times the sample volume, for 2 h to overnight.
Dialyze the sample against a solution that has the same concentrations of chaotropes (urea and thiourea) and DTT as the sample. Other more expensive solution components such as CHAPS and carrier ampholytes do not need to be included in the dialysis solution. These components may be added to their required concentrations following dialysis.
Samples for 2-D electrophoresis should be prepared in a solution that will be compatible with first-dimension IEF, including urea, CHAPS, and DTT.
Vivaspin
Vivaspin concentrators can also be used for desalting samples
若要繼續閱讀,請登入或建立帳戶。
還沒有帳戶?