推薦產品
等級
certified reference material
pharmaceutical secondary standard
品質等級
agency
traceable to USP 1477604
API 家族
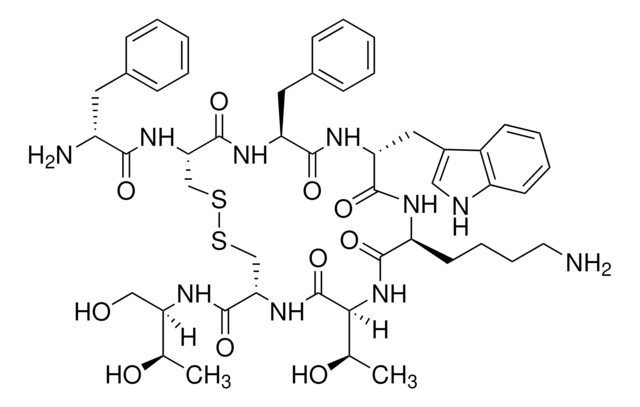
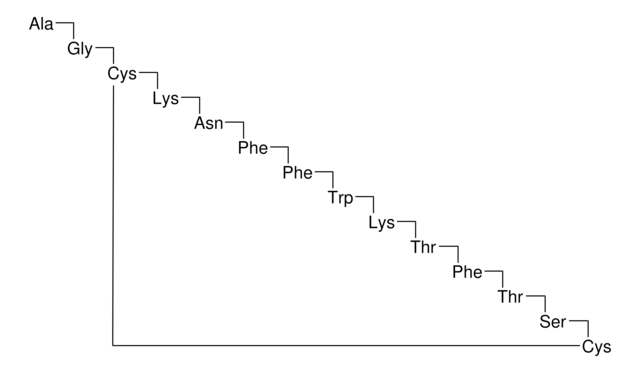
octreotide
形狀
powder
CofA
current certificate can be downloaded
包裝
pkg of 10 mg
應用
pharmaceutical
儲存溫度
-10 to -25°C
InChI
1S/C49H66N10O10S2.2C2H4O2/c1-28(61)39(25-60)56-48(68)41-27-71-70-26-40(57-43(63)34(51)21-30-13-5-3-6-14-30)47(67)54-37(22-31-15-7-4-8-16-31)45(65)55-38(23-32-24-52-35-18-10-9-17-33(32)35)46(66)53-36(19-11-12-20-50)44(64)59-42(29(2)62)49(69)58-41;2*1-2(3)4/h3-10,13-18,24,28-29,34,36-42,52,60-62H,11-12,19-23,25-27,50-51H2,1-2H3,(H,53,66)(H,54,67)(H,55,65)(H,56,68)(H,57,63)(H,58,69)(H,59,64);2*1H3,(H,3,4)/t28-,29?,34?,36?,37?,38?,39-,40?,41?,42?;;/m1../s1
InChI 密鑰
QWFYIFWTVZFPRY-AARKYNAGSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Octreotide belongs to the group of synthetic cyclic octapeptides, known for its selectivity towards inhibiting the growth hormone. It binds to the somatostatin receptor 2 (SSTR2), and thereby prevents the secretion pathways of growth hormone. Hence it is used in the treatment of diseases caused by overproduction of growth hormone, such as acromegaly.
應用
- Octreotide acetate analysis of amino acids including hydrolysis, derivatization of released amino acids with 4-N,N-dimethylaminoazobenzene-4ʹ-sulfonyl chloride (DABS-Cl), and finally their reversed phase-high performance liquid chromatography (RP-HPLC) determination
- Development of a quantitative nuclear magnetic resonance (1H-qNMR) based method for the estimation of octreotide acetate in bulk drug
- Determination of octreotide acetate in pharmaceutical formulations using a stability-indicating capillary zone electrophoresis method (CZE)
- Estimation of octreotide acetate from a peptide-based hydrogel using an ultra-high performance liquid chromatographic method in combination with photo-diode array detection (PDA), following the quality-by-design (QbD) approach
分析報告
腳註
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務