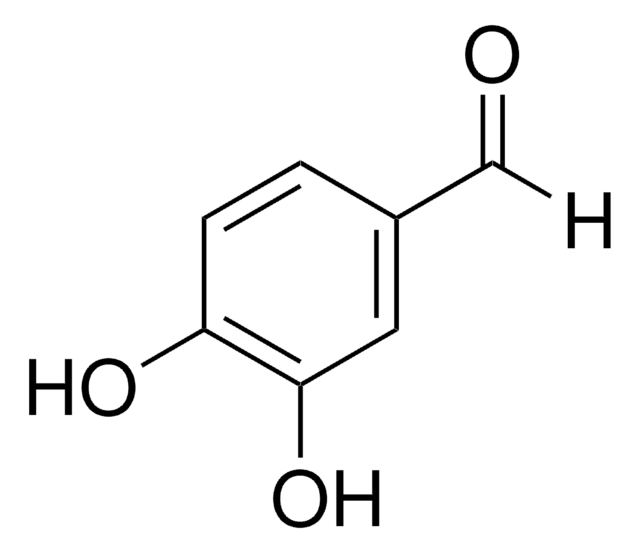
推薦產品
化驗
>98% (TLC)
形狀
powder
包裝
pkg of 1 × 1 mg (857393P-1mg)
製造商/商標名
Avanti Polar Lipids 857393P
脂質類型
sphingolipids
bioactive lipids
運輸包裝
dry ice
儲存溫度
−20°C
InChI
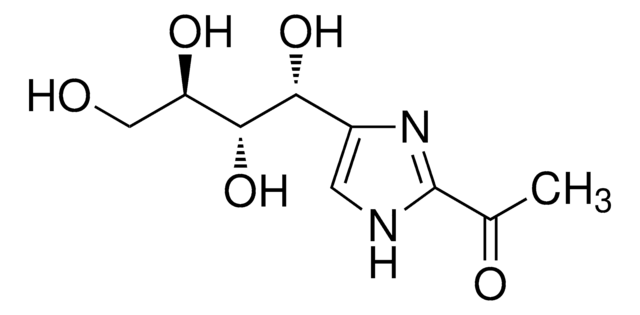
1S/C9H14N2O5/c1-4(13)9-10-2-5(11-9)7(15)8(16)6(14)3-12/h2,6-8,12,14-16H,3H2,1H3,(H,10,11)/t6-,7-,8-/m1/s1
InChI 密鑰
CQSIXFHVGKMLGQ-BWZBUEFSSA-N
一般說明
2-Acetyl-5-tetrahydroxybutyl imidazole (THI) is an inhibitor of sphingosine-1-phosphate lyase (S1P lyase).
Sphingosine-1-phosphate (S1P) lyase catalyzes the irreversible decomposition of S1P to hexadecanaldehyde and phosphoethanolamine. 2-Acetyl-5-tetrahydroxybutyl imidazole (THI) is an inhibitor of S1P lyase. Because S1P lyase is primarily expressed in lymphatic tissue, treatment of mice with THI (50 μg/ml in drinking water) increases lymphoid tissue S1P concentrations 100-fold, reducing lymphocyte egress from thymus and peripheral lymphoid organs. The resulting lymphopenia is reversible following cessation of THI treatment. Reducing S1P lyase activity results in therapeutic levels of immunosuppression without the non-lymphoid lesions that result from synthetic S1P receptor agonists.
生化/生理作用
2-Acetyl-5-tetrahydroxybutyl imidazole (THI) can block S1P lyase in vivo and up-regulates circulatory and tissue S1P levels. It has the ability to repress dystrophic muscle degeneration.
包裝
5 mL Amber Glass Screw Cap Vial (857393P-1mg)
法律資訊
Avanti Research is a trademark of Avanti Polar Lipids, LLC
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
Emigration of mature T cells from the thymus is inhibited by the imidazole-based compound 2-acetyl-4-tetrahydroxybutylimidazole.
Gugasyan R, et al.
Immunology, 93, 398-404 (1998)
The immunosuppressive compound 2-acetyl-4-tetrahydroxybutyl imidazole inhibits the allogeneic mixed lymphocyte reaction by sequestration of a recirculating subpopulation of T cells.
Bradbury MG, et al.
Immunology, 87, 80-85 (1996)
2-Acetyl-5-tetrahydroxybutyl imidazole (THI) protects 661W cells against oxidative stress
Fabiani C, et al.
Naunyn-Schmiedeberg'S Archives of Pharmacology, 390(7), 741-751 (2017)
Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients.
Schwab SR, et al.
Science, 309, 1735-1739 (2005)
Molecular mechanism of sphingosine-1-phosphate action in Duchenne muscular dystrophy
Nguyen TDH, et al.
Disease models & mechanisms, 7(1), 41-54 (2014)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務