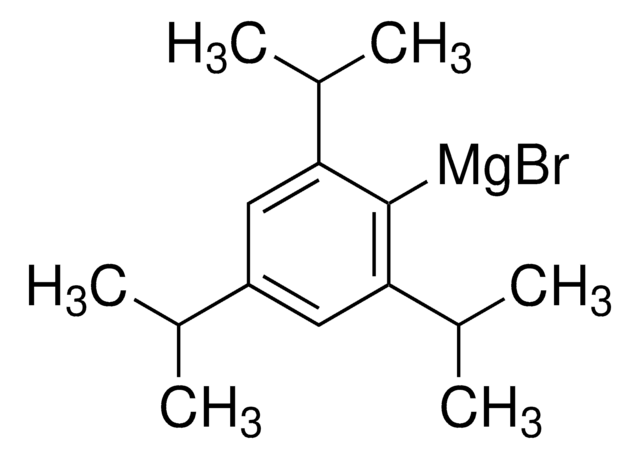
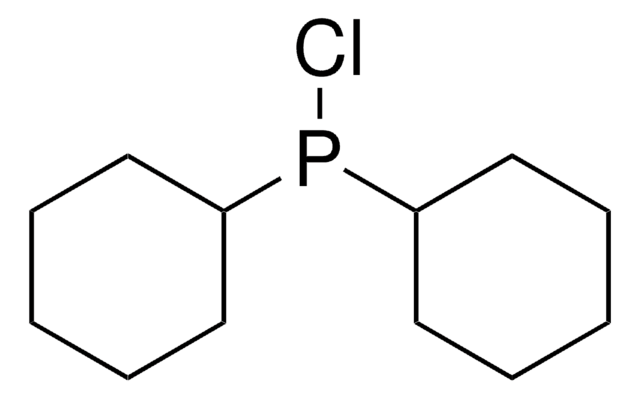
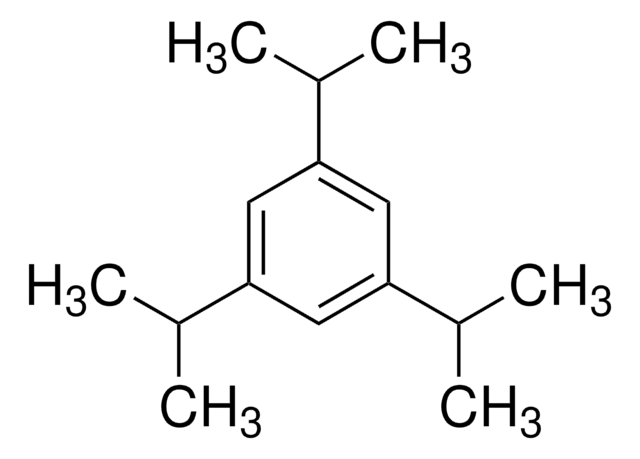
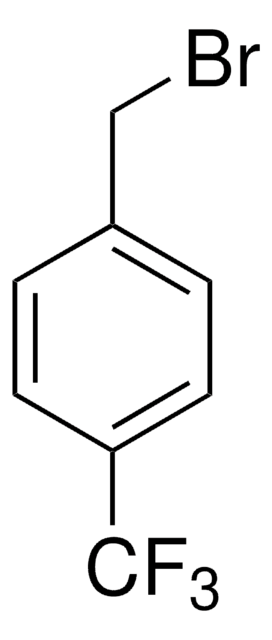
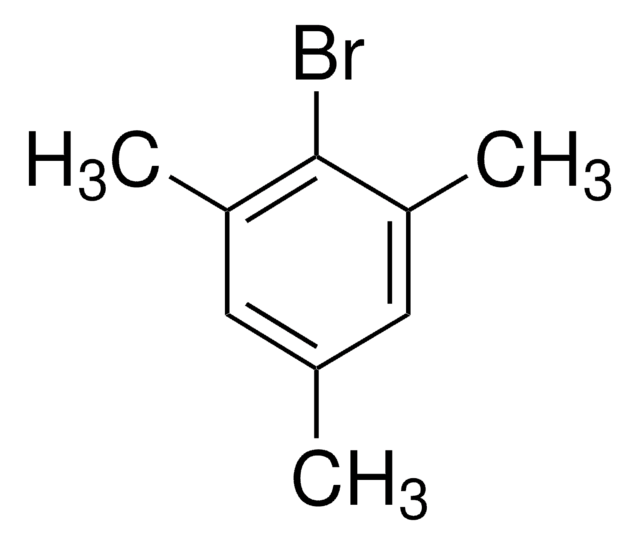
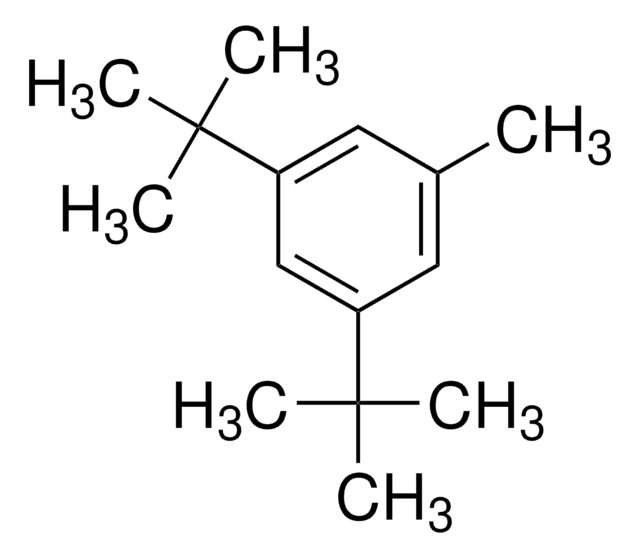
推薦產品
化驗
97%
mp
168-173 °C (lit.)
SMILES 字串
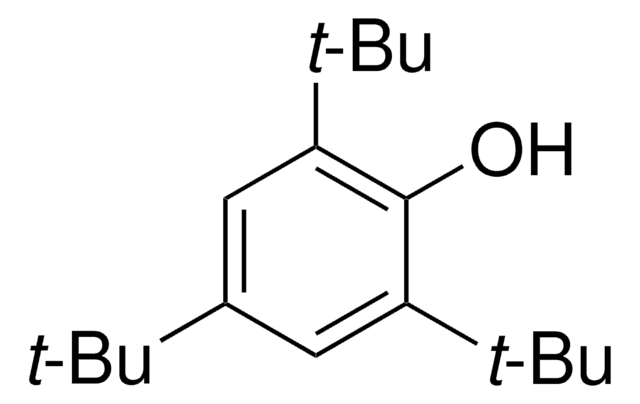
CC(C)(C)c1cc(c(Br)c(c1)C(C)(C)C)C(C)(C)C
InChI
1S/C18H29Br/c1-16(2,3)12-10-13(17(4,5)6)15(19)14(11-12)18(7,8)9/h10-11H,1-9H3
InChI 密鑰
JOKZWHPYNRDCOA-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
1-Bromo-2,4,6-tri-tert-butylbenzene (2,4,6-tri-tert-butylbromobenzene) is a hindered aryl bromide. 1-Bromo-2,4,6-tri-tert-butylbenzene on reaction with phenylboronic acid yields α,α-dimethyl-β-phenyl hydrostyrene.
應用
1-Bromo-2,4,6-tri-tert-butylbenzene was used in the synthesis of bulky biarylphosphine ligand. This ligand was reported to participate in the Pd-catalyzed C-O cross-coupling of a wide range of aryl halides and phenols under milder conditions. It was used to investigate the effect on oligomerization of increased steric bulk in dimethylindium(III) chalcogenolates. It may be used to form α,α-dimethyl-β-phenyl hydrostyrene by reacting with phenylboronic acid.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
The first Cu-and amine-free Sonogashira-type cross-coupling in the C-6-alkynylation of protected 2'-deoxyadenosine.
Ngassa FN, et al.
Tetrahedron, 65(21), 4085-4091 (2009)
Luca Salvi et al.
Organic letters, 14(1), 170-173 (2011-12-21)
A new bulky biarylphosphine ligand (L8) has been developed that allows the Pd-catalyzed C-O cross-coupling of a wide range of aryl halides and phenols under milder conditions than previously possible. A direct correlation between the size of the ligand substituents
Palladium(0)-catalyzed intermolecular amination of unactivated C(sp³)-H bonds.
Jun Pan et al.
Angewandte Chemie (International ed. in English), 50(37), 8647-8651 (2011-08-04)
Glen G Briand et al.
Dalton transactions (Cambridge, England : 2003), 39(16), 3833-3841 (2010-04-08)
The effect on oligomerization of increased steric bulk in dimethylindium(III) chalcogenolates (Me(2)InER') (E = O, S, Se) has been examined. The facile reaction of Me(3)In with a series of phenols, thiophenols and selenophenols afforded the compounds [Me(2)InO(C(6)H(5))](2) (1), [Me(2)InO(2,6-Me(2)C(6)H(3))](2) (2)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務