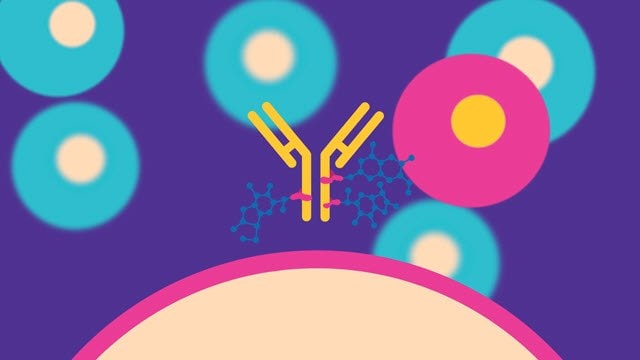
抗体药物偶联物(ADC)生产
抗体药物偶联 (ADC) 技术使用单克隆抗体(mAb)将有效的高活性药物成分(HPAPI)递送至靶细胞。偶联形式下的HPAPI会表现出选择性的细胞毒性,可使非靶细胞免受毒性影响。 然而,发掘ADC技术的巨大潜力棘手而复杂。为了表征分子并证明其纯度、均一性和稳定性,先进的生产套件和专用设备必不可少。将独特的肿瘤靶向mAb与有效杀伤细胞的细胞毒性小分子药物进行有效相连只是开始——为了满足ADC生产的需求,需要在小分子和大分子的开发、生产和检测方面拥有全面的产品组合和广泛的专业知识。 |
相关产品资源
分析开发和产品表征
文章:什么造就优异的ADC?
白皮书:了解您ADC的关键质量属性
网络研讨会:在ADC药物开发中使用正交分析的优势
网络研讨会:蝴蝶效应:如何洞悉微小变化对ADC的影响
网络研讨会:从概念到临床材料推进ADC项目的综合方法
海报:ADC上谨慎药物接头变量与结合活性之间的相关性
海报:World ADC SD 21:增强的数据分析让早期ADC筛选和候选药物选择成为可能
抗体、接头-有效载荷和偶联服务
手册:了解全面的ADC服务和解决方案
网络研讨会:借助完整供应链解决方案加快ADC开发
活页:通过ADC Express™临床前偶联服务筛选最优候选药物
白皮书:抗体药物偶联物的商品化:CMO之旅
接头-有效载荷技术
白皮书:单分散和活化PEG在ADC开发中的优势
活页:活页:用于您药物偶联项目的DM1 - Mertansine GMP质量有效载荷
活页:ADcore有效载荷中间体:简化您的有效载荷合成
网络研讨会:有效载荷核心产品线加速ADC临床申报时间表
网络研讨会:ChetoSensar™技术平台:解决溶解度问题
活页:ChetoSensar™
建立强大的ADC合作伙伴关系的关键包括从基因序列到最终药品稳定性测试的无缝供应链可通过最大程度降低开发和生产的复杂性来缩短上市时间。
|
工作流程
找到您的有效载荷供应中的缺失环节
A highly active payload and drug-linker are matched with the mAb for eventual conjugation into an antibody drug.
Monodisperse and activated PEGs, or Chetosensar™ technology may solve solubility challenges during drug discovery and development. Advanced payload intermediates for the most common classes of payloads can help to reduce development timelines.

通过偶联服务实现相互联结
The developed components of cell line, antibody, payload, and linker come together during conjugation and/or bioconjugation.
ADC原料药(BDS)测试服务
BDS as well as final drug product receive extensive analytical testing, including stability and release testing.
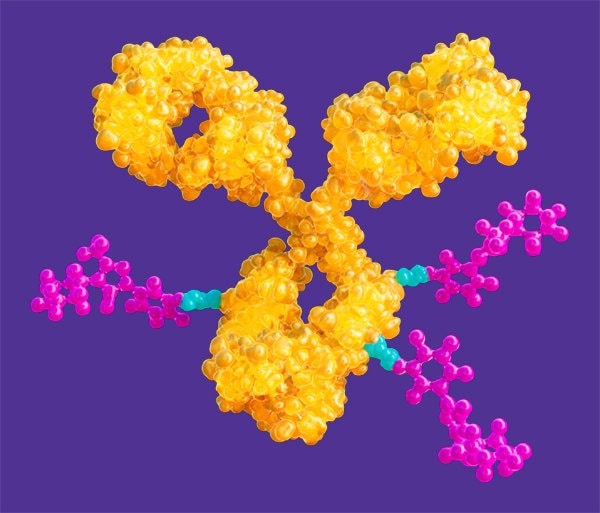
与细胞毒性分子偶联
Linkage between the antibody and a highly active payload is a critical component of an ADC, requiring a broad range of products and chemicals.
色谱(可选)
An optional chromatographic step can be used to remove high molecular weight species such as antibody aggregates and free drug residuals, while supporting optimization of drug-antibody-ratio (DAR) and control of polydispersity.
The Eshmuno® CMX resin has been designed for highly selective mixed mode chromatography.
超滤/渗滤
Removing residual solvent and free drug after conjugation is necessary prior to preparation of the final formulation with the desired concentration and buffer.
The Pellicon® Capsule is the first of its kind–a true single-use TFF device that comes ready to process ADCs in minutes.
无菌过滤
Sterilizing-grade filtration is under increasingly intense scrutiny by regulatory bodies, requiring a high degree of sterility assurance.
ADC BDS最终填充和制剂
The BDS is prepared for a final and unique formulation. The lyophilized dosage form is preferred and usually contains a buffer, stabilizer (e.g, trehalose or sucrose), and surfactant.
若要繼續閱讀,請登入或建立帳戶。
還沒有帳戶?