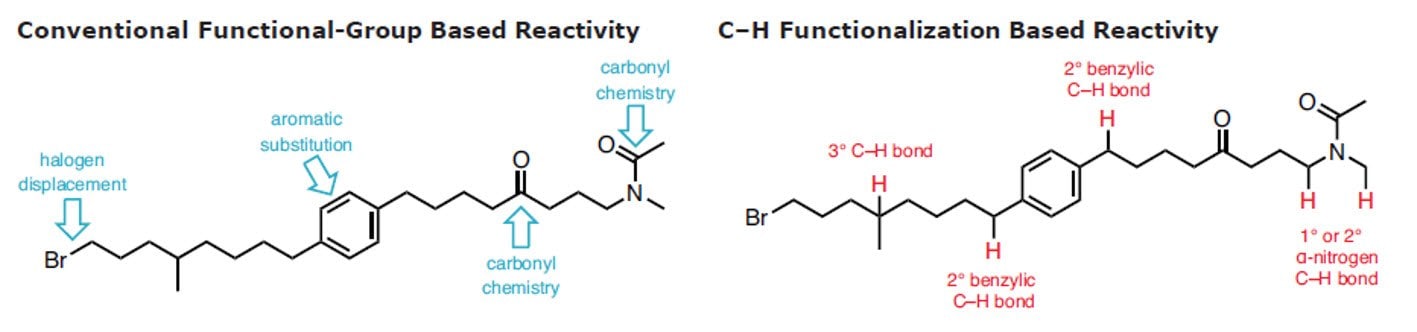
C-H官能团化
C-H官能团化被称为合成有机化学的最高目标。1 在有机化学、有机金属和催化领域的近期努力在了解C-H键的反应性和利用该洞见形成稳健反应方面取得了重大收获。这表明,是时候广泛地将这些策略引入逆合成字典中。2-11 以选择的、可控的方式将C–H可靠、可预测地转换为C–C、 C–N、 C–O 或 C–X 键有利于步骤经济和减少浪费。
用于C–H活化的新方法扩展了给定分子中可靶向的位点数量,从而增加了将其精细化为更复杂产品的机会。此外,它还可实现在有机合成中靶向完全不同类型的化学键,尤其是具有高化学选择性的化学键。通过结合传统的官能团化学,C-H官能团化极大简化了用于构建复杂天然产物和药物化合物的化学合成过程。尽管C-H官能团化具有明显的优势,12但许多有机化学课程尚未将这种方法更新进去,更多进一步的信息可在C-H官能团化手册中找到。
相关技术文章
- Professor Karl Anker Jørgensen and his group have developed ethers which serve as excellent chiral organocatalysts in the direct asymmetric α-functionalization of aldehydes.
- The synthesis of heteroaromatic and aromatic compounds is at the heart of the chemical industry. The ever-growing demand for new chemical entities, coupled with dwindling resources and time constraints allotted to any given research project, a rapid way to diversify (hetero)aromatic scaffolds is needed.
- The Du Bois group at Stanford University has made substantial progress within the field of Rh-catalyzed C–H amination via oxidative cyclization of carbamate, sulfamate, sulfamide, urea, and guanidine substrates to give 1,2- and 1,3-heteroatom motifs masked in the form of 5- and 6-membered ring heterocycles.
- Aryl chlorides are commonly used in cross-coupling reactions and can serve as key intermediates towards the synthesis of pharmaceutical drug candidates and natural products.
- A recyclable, ligand-free ruthenium catalyst for C–H activation reactions and concomitant C–C bond formation in the presence of water.
- 查看完整内容 (10)
参考文献
1.
Arndtsen BA, Bergman RG, Mobley TA, Peterson TH. 1995. 在均质溶液中通过合成金属复合物进行选择性分子间碳氢键激活. Acc. Chem. Res.. 28(3):154-162. https://doi.org/10.1021/ar00051a009
2.
He J, Wasa M, Chan KSL, Shao Q, Yu J. 2017. 钯催化的烷基C-H键转化. Chem. Rev.. 117(13):8754-8786. https://doi.org/10.1021/acs.chemrev.6b00622
3.
Wang D, Weinstein AB, White PB, Stahl SS. 2018. 配体促进的、钯催化的需氧氧化反应. Chem. Rev.. 118(5):2636-2679. https://doi.org/10.1021/acs.chemrev.7b00334
4.
Davies HML, Morton D. 2016. C-H官能团化的近期进展. J. Org.Chem.. 81(2):343-350. https://doi.org/10.1021/acs.joc.5b02818
5.
Upp DM, Lewis JC. 2017. 使用重新利用的或人工金属酶进行选择性的C-H键官能团化. 化学生物学的当前观点. 3748-55. https://doi.org/10.1016/j.cbpa.2016.12.027
6.
Cernak T, Dykstra KD, Tyagarajan S, Vachal P, Krska SW. 用于类药物分子后期官能团化的药物化学家工具箱. Chem. Soc.Rev.. 45(3):546-576. https://doi.org/10.1039/c5cs00628g
7.
Yamaguchi J, Yamaguchi AD, Itami K. 2012. C-H键官能团化:用于天然产物和药物的新兴合成工具. Angew.Chem. Int. Ed.. 51(36):8960-9009. https://doi.org/10.1002/anie.201201666
8.
Lyons TW, Sanford MS. 2010. 钯催化的配体定向C-H官能团化反应. Chem. Rev.. 110(2):1147-1169. https://doi.org/10.1021/cr900184e
9.
Wencel-Delord J, Dröge T, Liu F, Glorius F. 2011. 旨在温和的、金属催化的C-H键激活. Chem. Soc.Rev.. 40(9):4740. https://doi.org/10.1039/c1cs15083a
10.
Arockiam PB, Bruneau C, Dixneuf PH. 2012. 钌催化的C-H键激活和官能团化. Chem. Rev.. 112(11):5879-5918. https://doi.org/10.1021/cr300153j
11.
Engle KM, Mei T, Wasa M, Yu J. 2012. 作为用于形成广泛有用的官能团化反应的强而有力的工具的弱配体作用. Acc. Chem. Res.. 45(6):788-802. https://doi.org/10.1021/ar200185g
12.
Gutekunst WR, Baran PS. 2011. 全合成中的C-H官能团化逻辑. Chem. Soc.Rev.. 40(4):1976. https://doi.org/10.1039/c0cs00182a
登入以繼續
若要繼續閱讀,請登入或建立帳戶。
還沒有帳戶?