Aldehydes
Hartmann and co-workers have described the synthesis of a series of potent and selective inhibitors of aldosterone synthase (CYP11B2), in which the key synthetic step was a Wittig reaction using various heterocyclic aldehydes (Scheme 1). The isoquinoline adduct was a very potent and selective inhibitor of CYP11B2. The successful inhibition of CYP11B2 has been proposed as a strategy for treatment of congestive heart failure and myocardial fibrosis.1
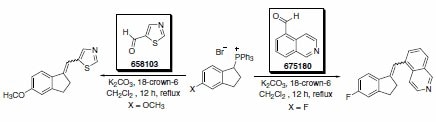
Scheme 1. (658103)(675180)
4-(1-Pyrrolidino)benzaldehyde was recently reported as a key building block in the synthesis of an improved inhibitor of NS5B polymerase of the hepatitis C virus (Scheme 2).2 The product from this substrate displayed significant improvement in the potency of the original HTS lead compound (Figure 1).
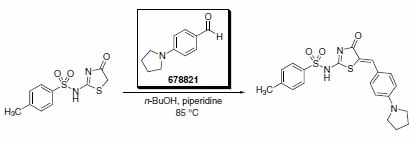
Scheme 2.(678821)
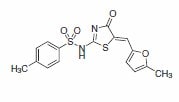
Figure 1.
Professor Jørgensen’s group has recently reported an asymmetric formal synthesis of (–)-paroxetine, a selective serotonin reuptake inhibitor used in the treatment of anxiety and other psychological disorders.3 The key step involved the organocatalytic conjugate addition of dibenzyl malonate to trans-4-fluorocinnamaldehyde (Scheme 3). Similarly, Wang’s research group has used the Jørgensen organocatalyst in a tandem Michael-aldol reaction to furnish chiral thiochromenes in excellent yields (Scheme 4).4
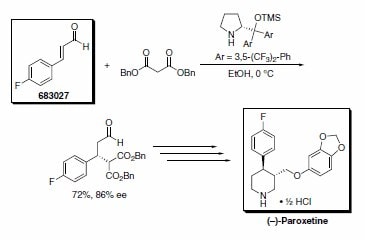
Scheme 3. (683027)
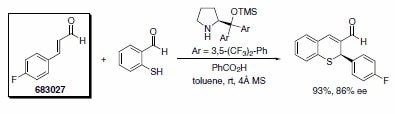
Scheme 4. (683027)
References
To continue reading please sign in or create an account.
Don't Have An Account?