34007
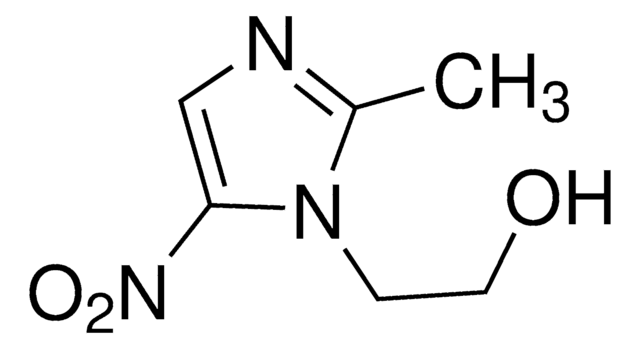
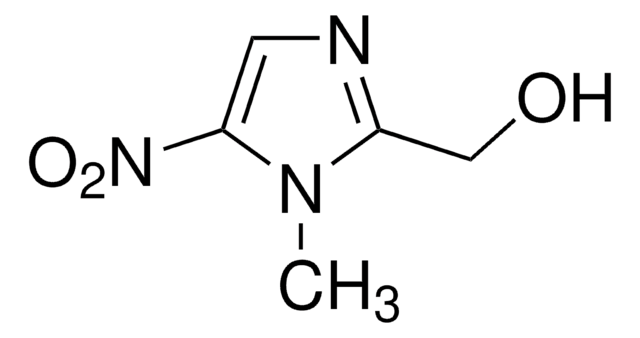
Metronidazole-OH
VETRANAL®, analytical standard
Synonym(s):
1-(2-Hydroxyethyl)-2-hydroxymethyl-5-nitroimidazole, Hydroxymetronidazole, MNZOH
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C6H9N3O4
CAS Number:
Molecular Weight:
187.15
MDL number:
UNSPSC Code:
41116107
PubChem Substance ID:
NACRES:
NA.24
Recommended Products
grade
analytical standard
Quality Level
product line
VETRANAL®
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
forensics and toxicology
pharmaceutical (small molecule)
format
neat
SMILES string
OCCn1c(CO)ncc1[N+]([O-])=O
InChI
1S/C6H9N3O4/c10-2-1-8-5(4-11)7-3-6(8)9(12)13/h3,10-11H,1-2,4H2
InChI key
AEHPOYAOLCAMIU-UHFFFAOYSA-N
General description
Metronidazole-OH is a genotoxic, carcinogenic and mutagenic drug, belonging to the class of nitroimidazoles, used as a broad spectrum veterinary drug for the treatment and prevention of certain bacterial and protozoal diseases in farm animals.
Application
Metronidazole-OH may be used as a reference standard for the determination of the antibiotic, metronidazole-OH in water samples and meat matrices using ultra-high-performance liquid chromatography coupled to quadrupole-linear ion trap tandem mass spectrometry (UPLC-Qtrap-MS/MS) and ultra-performance liquid chromatography (UPLC) coupled to time of flight mass spectrometry (TOF).
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
Recommended products
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
Legal Information
VETRANAL is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
C S Easmon et al.
The British journal of venereal diseases, 58(4), 246-249 (1982-08-01)
The hydroxy metabolite of metronidazole was found to be more active against 21 strains of Gardnerella vaginalis than the parent compound and less affected by culture in carbon dioxide. After 400 mg oral metronidazole (Flagyl) plasma concentrations of the two
Quantitative multiresidue method for about 100 veterinary drugs in different meat matrices by sub 2-particulate high-performance liquid chromatography coupled to time of flight mass spectrometry.
Kaufmann A, et al.
Journal of Chromatography A, 1194(1), 66-79 (2008)
M N Muscará et al.
British journal of clinical pharmacology, 40(5), 477-480 (1995-11-01)
Metronidazole pharmacokinetics were studied in patients with different degrees of liver cirrhosis, classified according to the Child-Pugh algorithm (A, B or C, as liver disease severity increases) and in schistosomic patients. Metronidazole (500 mg) was administered i.v. as a slow
Fast and comprehensive multi-residue analysis of a broad range of human and veterinary pharmaceuticals and some of their metabolites in surface and treated waters by ultra-high-performance liquid chromatography coupled to quadrupole-linear ion trap tandem mass spectrometry.
Gros M, et al.
Journal of Chromatography A, 1248(6), 104-121 (2012)
S L Pendland et al.
Antimicrobial agents and chemotherapy, 38(9), 2106-2110 (1994-09-01)
Metronidazole is metabolized to two major oxidative products: an acid metabolite and a hydroxy metabolite. While the activity of the acid metabolite is negligible, the activity of the hydroxy metabolite is approximately 65% of the activity of the parent drug.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service