523976
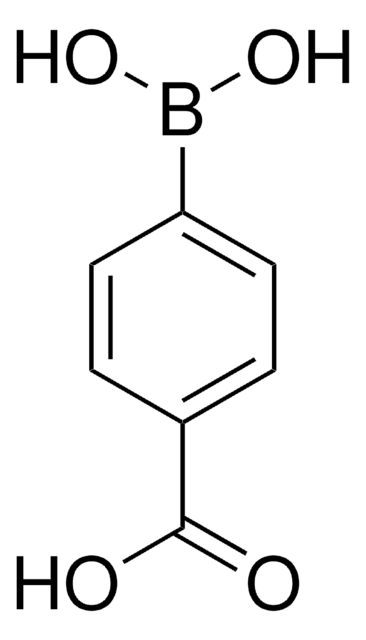
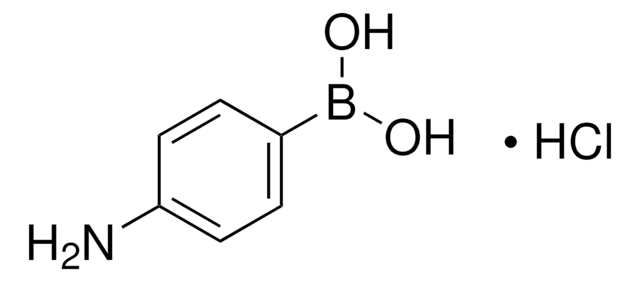
4-Hydroxyphenylboronic acid
≥95.0%
Synonym(s):
(p-Hydroxyphenyl)boronic acid, 4-Hydroxybenzeneboronic acid, p-hydroxy-benzeneboronic acid
About This Item
Assay
≥95.0%
form
solid
mp
>230 °C (lit.)
SMILES string
OB(O)c1ccc(O)cc1
InChI
1S/C6H7BO3/c8-6-3-1-5(2-4-6)7(9)10/h1-4,8-10H
InChI key
COIQUVGFTILYGA-UHFFFAOYSA-N
Related Categories
Application
- Suzuki-Miyaura coupling and Stille coupling reactions.
- Palladium-catalyzed aminocarbonylation and cross-coupling reactions.
- Suzuki reaction for preparation of bio-supported palladium nanoparticles as phosphine-free catalysts.
- Cu2O-catalyzed aerobic oxidative cross-coupling of tetrazoles.
It can also be used to prepare/promote:
- PDK1 inhibitory activity (cancer cell growth, survival, and tumorigenesis inhibitor).
- Rod-like dendronized polymers containing G4 and G5 ester dendrons via macromonomer approach by living ROMP.
- Estrone-derived cyclopamine analogs as Sonic Hedgehog signaling inhibitors for anti-cancer chemotherapeutics.
- Enzymatic inhibitors for the treatment of Gram-negative bacterial infections.
- Oligoarenes by Suzuki-Miyaura palladium-catalyzed cross-coupling.
Other Notes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service