Highly Potent APIs

High Potent API Manufacturing
As a seasoned contract manufacturer of highly potent active pharmaceutical ingredients (HPAPIs) with over three decades of experience, we are at the forefront of the industry and possess the expertise required to handle the distinct challenges that come with these powerful compounds. We serve as your capable and reliable CDMO partner, providing you with a range of scale and handling capabilities down to single-digit nanogram containment.
- Our facilities are certified by SafeBridge®, an industry leader in HPAPI handling certification, ensuring the utmost safety and security in all our operations.
- We offer an extensive portfolio of payload and advanced payload intermediate products that can accelerate your drug development.
Our commitment to quality and regulatory standards extends from pre-clinical to commercial programs, and our global supply chain network ensures the highest quality of raw materials sourcing. We seamlessly integrate with downstream ADC/bioconjugation service offers, providing you with end-to-end solutions for your pharmaceutical needs.

Safety
As one of the first facilities to receive SafeBridge® certification, we have been industry leaders in safe handling of Highly Potent APIs (HPAPIs) for over 30 years. To continue to build upon that pioneering spirit, we have invested heavily in our new Verona, Wisconsin HPAPI facility which boasts 6 new kilo labs with single-digit nanogram occupational exposure limit (OEL) containment. This innovative facility design raises the global standard for the safe handling of the industry’s most potent APIs.
Drug Development Acceleration Products
Accelerate your drug development efforts with our comprehensive product line of payloads and advanced payload intermediates. These products add value to your program by reducing development costs, decreasing supply chain complexity, and increasing speed of synthesis through reduction of required reaction steps – together accelerating your speed to clinic.
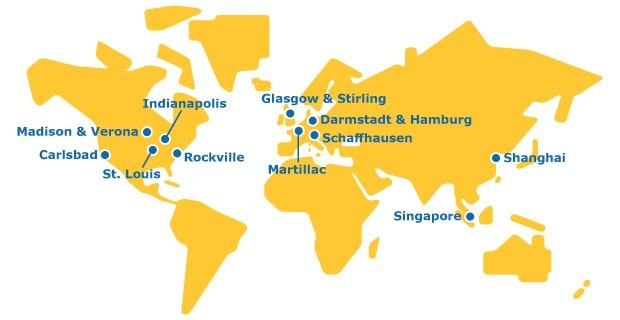
INTEGRATED SUPPLY CHAIN FOR HPAPIS AND ADCS
The global footprint of our HPAPI contract manufacturing services assures you wide-ranging regulatory and supply expertise, easily accessed through a single trusted partner.
Our API and HPAPI manufacturing sites are SafeBridge certified. Continued investment and expansion in these facilities build upon our decades of high potency expertise and makes us one of the largest single-digit nanogram OEL CTDMO providers in the world.
GMP contract manufacturing services for ADC and Bioconjugation, and Small Molecule bio-organics.
ISO 9001:2008 certified site meeting customer needs from development to commercial‑scale production of registered starting material.
Related Technical Resources
- Use of Payload Core Compounds to Accelerate ADC Clinical Development Timelines
In this whitepaper, we explore the complexities of developing ADCs and compare the conventional approach to starting with the payload core compounds.
- Overcoming the Solubility Challenges of Antibody-Drug Conjugates
In this flyer, we learn how to increase solubility of ADCs when incorporated into the linker-payload construct.
- Approaching the Nanogram Limit
Webinar discusses high potent API manufacturing evolution, safety concepts, and overcoming challenges in the industry.
- ADC Payload Intermediates and virtual HPAPI Site Tour
In this webinar, ADCs and other targeted therapies are opening up a world of exciting opportunities to change the nature of cancer treatment. HPAPIs are a critical element of these therapies.
- How to Accelerate and Enhance ADC Therapies
In this webinar, we discuss the overview of new products and services to accelerate and enhance ADC therapies, such as the ADCore payload, ChetoSensar™, and ADC Express™ service.
To continue reading please sign in or create an account.
Don't Have An Account?