Workflow for the Analysis of Polysorbate 80 in Erbitux® Formulation
Protocol for sample preparation and reversed phase HPLC-ELSD analysis of a non-ionic surfactant in a monoclonal antibody formulation
Dr. Stephan Altmaier, Chemistry R&D, Merck KGaA, Darmstadt, Germany

Sample preparation
Standardization & Calibration
Chromatography
Measurement & Analysis
ABSTRACT
A complete reversed phase HPLC-ELSD workflow has been developed for the quantification of polysorbate 80 (Tween® 80) in antibody formulations. In detail, it includes:
- Solid phase extraction (SPE) sample preparation procedure
- Preparation of calibration solutions
- Reversed phase HPLC-ELSD method for quantitative analysis of surfactant concentration
INTRODUCTION TO POLYSORBATE 80 ANALYSIS IN mAb FORMULATIONS
Monoclonal antibodies [mAbs or immunoglobulins (IgGs)] are large glycoproteins with a molecular weight of approximately 150 kDa (150,000 g/Mol). They are composed of two identical light chains (LC, molecular weight ca. 25 kDa each) and two identical heavy chains (HC, molecular weight ca. 50 kDa each) linked through covalent inter- and intra-chain disulfide bonds. They are utilized for the treatment of various types of cancer, and other diseases such as multiple sclerosis, Alzheimer’s disease, or migraine.
Careful and thorough characterization of therapeutic mAbs is essential for ensuring drug safety and efficacy. mAbs are typically manufactured in mammalian host cell lines in bioreactors, generating a large number of heterogeneous drug molecules. Establishing a number of critical quality attributes (CQAs) for each mAb and demonstrating that production batches are within acceptable limits is a requirement for both innovator and biosimilar therapeutics.1,2
Polysorbate 80 (PS 80; commercial name: Tween® 80) is a non-ionic surfactant that is utilized as a stabilizing excipient in protein therapeutics. PS 80 stabilizes proteins, prevents aggregation and nonspecific adsorption of primary and secondary antibodies to surfaces, reduces the rate of protein denaturation and increases the drug solubility and stability.3,4 In order to ensure product quality, the accurate quantitation of PS 80 in the final drug product is crucial. Figure 1 displays the chemical structure of polysorbate 80.

Figure 1.Chemical structure of polysorbate 80 (PS 80; commercial name: Tween® 80).
This report describes the application of reversed phase HPLC-ELSD (high performance liquid chromatography - evaporative light scattering detection) for the quantification of polysorbate 80 in an Erbitux® antibody drug formulation (Erbitux® is the trade name of the drug formulation using the monoclonal antibody cetuximab). Sample preparation is performed using solid phase extraction and a set of seven calibration solutions is prepared for system calibration.
EXPERIMENTAL PROCEDURE
The samples were received as Erbitux® drug product (DP, formulation of 5 mg/mL cetuximab, excipients: sodium chloride, glycine, polysorbate 80, citric acid monohydrate, sodium hydroxide, water) and were stored at 8 °C. Prior to sample preparation the samples were heated up to room temperature. A set of two Erbitux® DP batches was analyzed in this work. The polysorbate 80 concentration of the samples was approximately 0.1 mg/mL. No dilution was required before loading onto the solid phase extraction (SPE) cartridge. All solvents applied during sample preparation were of gradient grade HPLC quality or higher.
SAMPLE PREPARATION
The blank sample was represented by pure water and does not undergo the solid phase extraction process. The Erbitux® DP samples were purified by SPE. In detail, sample preparation was executed as follows:
- 4M Guanidinium hydrochloride solution
Dilute 80 mL guanidinium hydrochloride solution 6M with 40 mL water. - 10% Methanol
Dilute 10 mL methanol with 90 mL water to obtain a solution of 10% methanol in water. - Sample preparation – Solid phase extraction
- Position Supel™ Swift HLB SPE cartridge in Visiprep™ SPE Vacuum Manifold.
- Prime with 1 mL methanol.
- Condition with 1 mL water.
- Load 0.5 mL of an Erbitux® DP sample solution, add 0.5 mL water.
- Wash with 1 mL 4M guanidinium hydrochloride solution.
- Wash with 1 mL 10% methanol.
- Elute with 1 mL acetonitrile and collect eluent in 15 mL centrifuge tube.
- Repeat elution once and collect eluent in the same centrifuge tube.
- Evaporate acetonitrile in vacuum rotary evaporator at 40 °C for 25 minutes.
- Reconstitute sample with 250 µL water, vortex mix well and then transfer to HPLC glass vials.
STANDARD PREPARATION
The preparation of calibration standards was performed as follows:
- PS 80 standard stock solution 1.2 mg/mL
For the preparation of PS 80 stock solution (c = 1.2 mg/mL) weigh approximately 60 mg PS 80 into a 50 mL volumetric flask and fill up to mark with water. - PS 80 calibration standards
Prepare dilution series according to Table 1 to obtain a set of seven calibration standards.
RP-HPLC-ELSD SYSTEM SETUP
The essential settings of the Hitachi Chromaster chromatography system and the gradient conditions applied in the analysis of polysorbate 80 are listed in Tables 2 and 3 below.
DATA ANALYSIS
Data was processed with Chromeleon™ 7.2.10 software; due to application of a gradient profile and the necessity to summarize peak areas in the retention time range from approximately 6 to 9 minutes, the integration was executed manually. Integration of peaks outside the mentioned range was inhibited automatically. The calibration type applied was “Quad with offset”.
CALIBRATION DATA
A total of seven polysorbate 80 calibration standards was prepared. Quadratic regression revealed an excellent fit of the resulting calibration curve over the entire calibration range, with an R2 value of 0.9997 (see Figure 2). Experimental data obtained from the calibration experiments are listed in Table 4. Figure 3 displays an overlay of the chromatograms obtained by the analysis of the seven calibration standards.

Figure 2. HPLC-ELSD calibration curve obtained by injection of polysorbate 80 calibration standards 1-7.
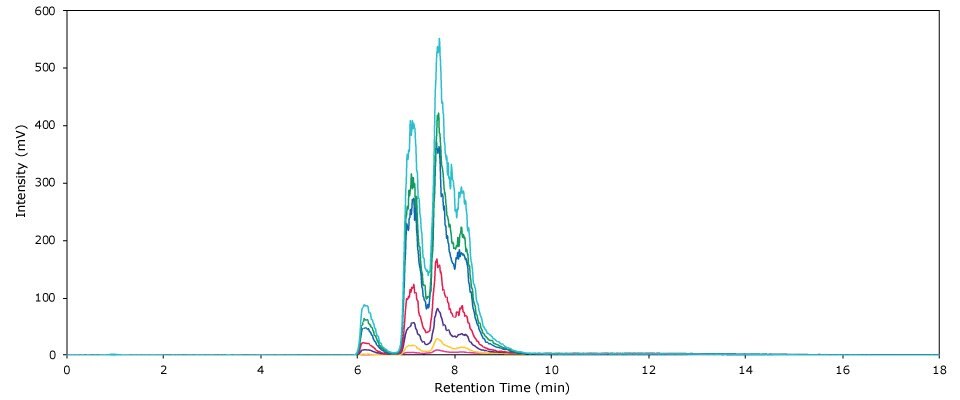
Figure 3 .Overlay of chromatograms obtained by the HPLC-ELSD analysis of all seven PS 80 calibration standards.
RESULTS
In this work, an Ascentis® Express C18 HPLC column was utilized for the HPLC-ELSD analysis of polysorbate 80 in two different batches of an Erbitux® antibody drug formulation. The HPLC column applied is comprised of reversed phase-modified, superficially porous silica particles and enables a fast, high-performance analysis. Sample preparation by hydrophilc-lipophilic balanced solid phase extraction was shown to effectively separate PS 80 from major amounts of drug product excipients. Duplicates of a total of five samples of each of the batches B4G and BM9 were analyzed to determine their polysorbate 80 content (see also Table 5). The corresponding ELSD traces of two representative samples of each batch are shown in Figure 4.
The analysis results revealed a PS 80 content of the samples of 0.12 and 0.14 mg/mL, which is in line with typical surfactant concentrations in antibody drug formulations. The calibration curve displayed an excellent quadratic fit over the entire calibration range, with an R2 value of 0.9997, and the LOD for the HPLC-ELSD method was 0.0055 mg/mL.
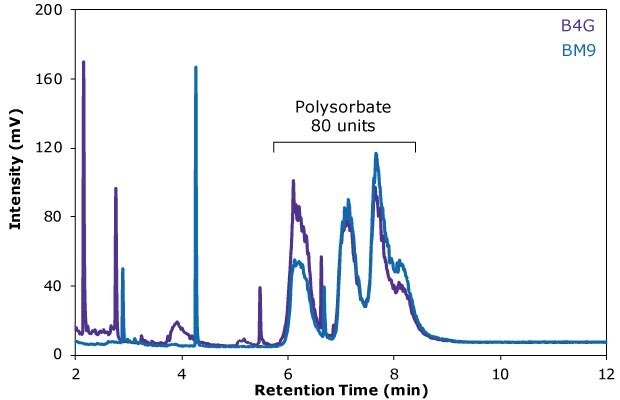
Figure 4 .RP-HPLC-ELSD chromatogram of the Erbitux® antibody drug samples B4G (purple trace) and BM9 (blue trace). Several polysorbate 80 peaks are visible in the range from approximately 6 to 9 minutes.
CONCLUSION
This report describes the entire workflow for the quantitative analysis of polysorbate 80 in two Erbitux® antibody drug formulations, using reversed phase HPLC-ELSD analysis. An Ascentis® Express C18 HPLC column packed with superficially porous silica particles was applied for the separation of PS 80 and matrix compounds.
The workflow includes a sample purification process using solid phase extraction with HLB cartridges and subsequent analysis of the samples by reversed-phase HPLC-ELSD. HPLC system calibration data was obtained by the preparation and analysis of seven polysorbate 80 standard solutions and allows for a simple quantification of polysorbate 80 content in mAb samples.
The chromatographic method established is suitable for sample separation and analysis of PS 80 and can also be applied in the quantification of similar non-ionic surfactants.
References
To continue reading please sign in or create an account.
Don't Have An Account?