D1797
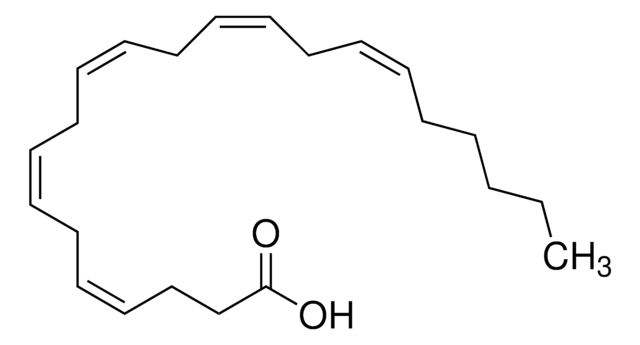
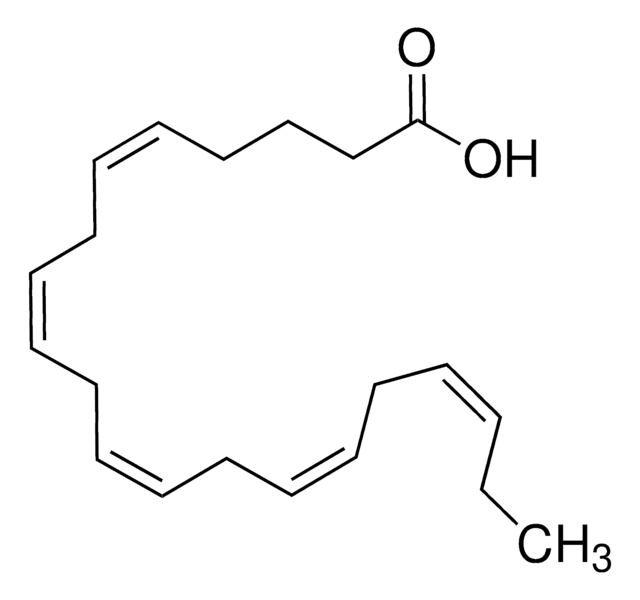
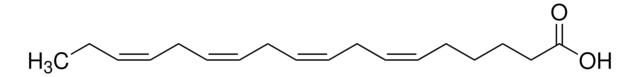
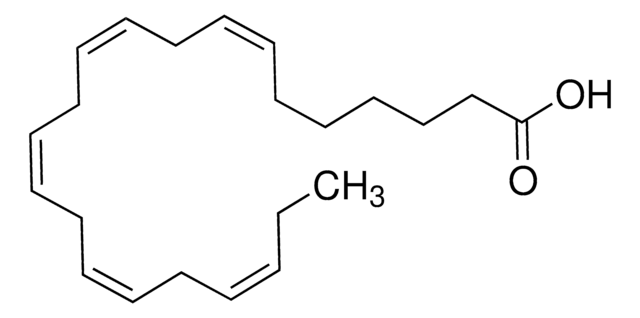
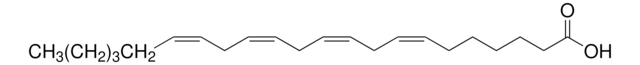
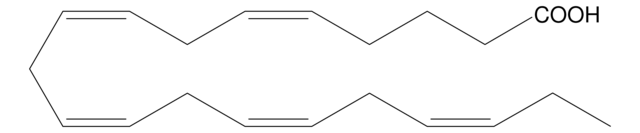
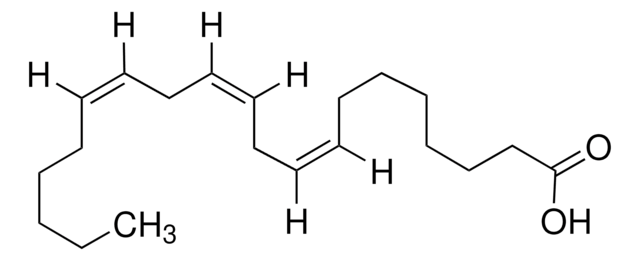
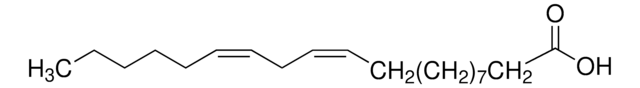
all-cis-7,10,13,16,19-Docosapentaenoic acid
synthetic, ≥97%
Synonym(s):
Clupanodonic acid
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(1)
Select a Size
Change View
About This Item
Empirical Formula (Hill Notation):
C22H34O2
CAS Number:
Molecular Weight:
330.50
MDL number:
UNSPSC Code:
12352211
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
biological source
synthetic
Quality Level
Assay
≥97%
form
liquid
functional group
carboxylic acid
lipid type
unsaturated FAs
shipped in
ambient
storage temp.
−20°C
SMILES string
CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCCCC(O)=O
InChI
1S/C22H34O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22(23)24/h3-4,6-7,9-10,12-13,15-16H,2,5,8,11,14,17-21H2,1H3,(H,23,24)/b4-3-,7-6-,10-9-,13-12-,16-15-
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jan Philipp Schuchardt et al.
Prostaglandins, leukotrienes, and essential fatty acids, 121, 76-87 (2017-06-28)
EPA and DHA cause different physiological effects, which are in many cases mediated via their oxidative metabolites (oxylipins). However, metabolism studies investigating the effect of either EPA or DHA on comprehensive oxylipin patterns are lacking. The short and long term
A M Eltweri et al.
Clinical nutrition (Edinburgh, Scotland), 36(3), 768-774 (2016-06-28)
It has been demonstrated that short term intravenous (IV) administration of omega-3 polyunsaturated fatty acids (PUFAs) is more effective than oral supplementation at promoting incorporation of the bioactive omega-3 PUFAs eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) into plasma, blood
Additional data from our study on fatty acids variations during lactation: correlations between n-3 and n-6 PUFAs in human colostrum, transitional, and mature milk.
Carolina Moltó-Puigmartí et al.
Clinical nutrition (Edinburgh, Scotland), 30(3), 402-403 (2011-04-08)
Yuko Yonezawa et al.
International journal of molecular medicine, 18(4), 583-588 (2006-09-12)
We reported previously that unsaturated linear-chain fatty acids of the cis-configuration with a C18-hydrocarbon chain such as linoleic acid (cis-9, 12-octadecadienoic acid, C18:2) could potently inhibit the activity of mammalian DNA polymerases (Biochim Biophys Acta 1308: 256-262, 1996). In this
A Voss et al.
The Journal of biological chemistry, 266(30), 19995-20000 (1991-10-25)
The hypothesis that the last step in the biosynthesis of 4,7,10,13,16,19-22:6 from linolenate is catalyzed by an acyl-CoA-dependent 4-desaturase has never been evaluated by direct experimentation. When rat liver microsomes were incubated with [1-14C]7,10,13,16,19-22:5, under conditions where linoleate was readily
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service