BugBuster® and Benzonase® Reagents are the Clear Solutions to Simple, Efficient Extraction of E. coli Proteins
Anthony Grabski, Mark McCormick, Robert Mierendorf
Novagen, Inc.
There are a variety of methods for cellular disintegration and extraction of proteins from E. coli ranging from enzymatic digestion and osmotic shock to ultrasonication and pressure disruption. Each method has inherent advantages and disadvantages. Generally, vigorous mechanical treatments reduce viscosity but can result in heat and oxidative inactivation of labile proteins, while gentle treatments may not release the target protein from the cells, and resulting extracts are extremely viscous. In addition to proteins, bacterial extracts contain large amounts of nucleic acid, ribosomal material, and dispersed cell wall polysaccharide resulting in a viscous gum-like mass. This viscosity is problematic during the initial steps of downstream processing.
Extraction and recovery of proteins expressed in E. coli has been simplified through the application of two powerful bioprocessing reagents: BugBuster® Protein Extraction Reagent and Benzonase® Nuclease. BugBuster® reagents are formulated for the gentle disruption of the E. coli cell wall, resulting in the liberation of soluble proteins. It provides a simple, rapid, low cost alternative to mechanical disruption methods such as French press or sonication for releasing target proteins in preparation for functional studies or purification. The proprietary formulation utilizes a mixture of non-ionic detergents that are capable of cell wall perforation without denaturing soluble proteins.
The Benzonase® reagent is a genetically engineered endonuclease produced and purified from E. coli strain W3110, a mutant of strain K12, containing the proprietary pNUC1 production plasmid.1,2 The plasmid encodes an endonuclease classified as a nonspecific phosphodiesterase originally isolated from Serratia marcescens.3,4 Structurally, the protein is a dimer of identical 245 amino acid, ~30 kDa subunits with two essential disulfide bonds.5–8 This promiscuous endonuclease attacks and degrades all forms of DNA and RNA (single stranded, double stranded, linear and circular) and is effective over a wide range of operating conditions (Table 1, ref. 9). The enzyme completely digests nucleic acids to 5'-monophosphate terminated oligonucleotides 2–5 bases in length.4,10 Although the nuclease is capable of cleavage at nearly all positions along a nucleic acid chain, sequence dependent preferences have been demonstrated.11 The enzyme prefers GC-rich regions in dsDNA while avoiding d(A):d(T)-tracts. Benzonase® reagent is available in two grades, Purity > 99% and Purity > 90%. Both preparations possess exceptionally high specific activities and are supplied free from measurable protease activities and viral contaminants. Benzonase® reagent is ideal for a wide variety of applications where complete digestion of nucleic acids is desirable. This article demonstrates the combination of BugBuster® and Benzonase® reagents as protein purification tools to extract recombinant proteins from E. coli and to reduce the viscosity of the resulting extract.
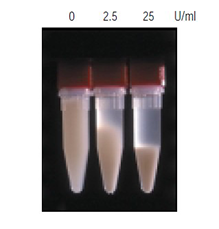
Figure 1.Viscosity reduction by Benzonase nuclease E. coli cells BL21(DE3) containing a pET construct were suspended in BugBuster® reagent (5 mL/g wet weight). Aliquots of the suspension were treated with the indicated amounts of Benzonase® nuclease for 10 min at room temperature, centrifuged at 350 × g for 3 min and photographed.
Extract preparation
The E. coli cells BL21(DE3) containing a pET construct encoding a His•Tag® fusion protein were grown in liquid culture, induced with IPTG for 5 h, harvested by centrifugation, and cell pellets frozen before BugBuster + Benzonase reagents extraction. Frozen cell pellets were completely resuspended at room temperature in BugBuster® reagent (5 mL/g wet weight) containing 0.5 mM PMSF and 15 µg/mL benzamidine. The crude suspension was divided into two equal aliquots. One aliquot was treated with 1 µL (25 units) Benzonase® nuclease (Purity > 90%) per mL of BugBuster® reagent used in the initial resuspension. The second control aliquot was not treated with Benzonase® nuclease. These crude extracts were reacted on a rotary mixer for 15–20 min at room temperature. Insoluble cell debris was removed by centrifugation at 16,000 × g for 20 min at 4 °C. The soluble supernatant fractions were carefully removed with a pipet and saved for purification of the target protein. The insoluble pellets were discarded.
Viscosity reduction
The results of Benzonase® nuclease treatment and its influence on viscosity reduction and nucleic acid digestion are clearly demonstrated in Figures. 1 and 2, respectively. Figure 1 demonstrates viscosity reduction through a simple centrifugation experiment employing 0, 2.5, and 25 units of Benzonase®/mL and BugBuster® reagents extract of E. coli cells. Benzonase® nuclease treatment significantly reduced the viscosity of crude cell extracts, allowing effective clarification with lower centrifugal force than is required to clarify untreated extract. In Figure 1, significant clarification of the extracts was seen with only 350 × g centrifugal force. Typically, untreated E. coli extracts are clarified by centrifugation at 20,000–50,000 × g in order to pellet cell wall fragments and membranes. After Benzonase® nuclease reatment, extracts can be clarified by centrifugal forces of only 5,000–10,000 × g. The lower centrifugal forces necessary to clarify Benzonase® nuclease treated cell extracts are more adaptable to largescale processes where generation of high centrifugal forces is impractical. Additionally, soluble protein supernatant yields are greater with Benzonase® nuclease treatment, because the precipitated materials form tight pellets upon centrifugation and less liquid is trapped than in the jelly-like pellets from untreated samples.
Digestion of nucleic acids
Figure 2 demonstrates the digestion of nucleic acids by Benzonase® nuclease from BugBuster® reagent extract of E. coli by agarose gel analysis and ethidium bromide staining. The Benzonase®nuclease titration series allows visual confirmation of progressive attack on nucleic acids and complete digestion as treatment levels increase from left to right. Digestion of the nucleic acids is the key to the effectiveness of Benzonase® reagent as a bioprocessing reagent for viscosity reduction.

Figure 2. Nucleic acid digestion by Benzonase® nuclease.The E. coli cells BL21(DE3) containing a pET construct were suspended in BugBuster® reagent (5 mL/g wet weight). Aliquots of the suspension were treated with the indicated amounts of Benzonase® nuclease for 30 min at room temperature. Samples were clarified by centrifugation and analyzed by agarose gel electrophoresis and ethidium bromide staining.

BugBuster® + Benzonase® reagents

BugBuster® reagent only
Figure 3. Analysis of BugBuster® + Benzonase® reagents extracted and IMAC purified His•Tag® protein. Samples were prepared and immobilized-metal affinity chromatography (IMAC) was performed as described in the text. The indicated samples were analyzed by SDS-PAGE on a 4–20% gradient gel stained with Coomassie blue.
Immobilized-metal affinity chromatography using His•Bind® Resins
Soluble proteins from the centrifuged extracts were applied by gravity flow to glass columns packed with His•Bind® Resin or Ni-NTA His•Bind® Resin (1.5 × 2.3 cm, ~4 mL bed volume). Unbound and weakly adsorbed proteins were washed from the columns with 10 column volumes of 20 mM Tris-HCl, pH 7.9 containing 5 mM imidazole and 0.5 M NaCl followed by 10 column volumes of 20 mM Tris-HCl, pH 7.9 containing 30 mM imidazole and 0.5 M NaCl. The His•Tag® fusion protein was eluted with five column volumes 20 mM TrisHCl, pH 7.9 containing 0.5 M imidazole and 0.5 M NaCl, and 1.5 mL fractions were collected. Fractions were analyzed and pooled according to densitometric scanning analysis of SDS-PAGE results and protein concentration determined by the BCA assay.12 Fractions 3–11 were pooled from each IMAC separation.
Results of the IMAC purification are shown in Table 2 and in Figure 3. Benzonase® nuclease treatment of the extract resulted in much higher column flow rates, higher product recovery, and greater product purity with both His•Bind® and Ni-NTA His•Bind® Resins, as compared to purification from extracts that had not been treated with Benzonase® nuclease.
Summary
Through the application of BugBuster® Protein Extraction Reagent and Benzonase® Nuclease, it is now possible to rapidly and efficiently extract and recover proteins expressed in E. coli without exposing them to the harsh conditions associated with ultrasonication and pressure disruption. Gentle release of intracellular proteins is achieved with BugBuster® reagent and extract viscosity reduced or eliminated by digestion of problematic nucleic acids with Benzonase® nuclease. The addition of Benzonase® nuclease for viscosity reduction and nucleic acid digestion provides several advantages over BugBuster® reagent treatment alone or other cellular disintegration techniques. Downstream processing advantages demonstrated independently and by these experiments include:
- Decreased centrifugal forces required for extract clarification
- Increased column flow rates and chromatographic performance13–16
- Higher yields of protein/product13–16
- Reduced processing time
- Increased biomass content and fewerhomogenization cycles for extracts processed by expanded bed adsorption17
- Degradation of nucleic acids fromrecombinant protein samples, enabling compliance with FDA guidelines for nucleic acid contamination9
Finally, Benzonase® nuclease digestion for nucleic removal is superior to traditional extraction or precipitation methods, which can reduce yield due to protein denaturation and coprecipitation. While DNase and RNase have also been used for viscosity reduction, Benzonase® nuclease has a higher specific activity, digests all forms of DNA and RNA, and has been genetically engineered and produced in bulk quantities specifically for biotechnological and biopharmaceutical applications.
References
如要继续阅读,请登录或创建帐户。
暂无帐户?