mRNA Synthesis for the Development of Vaccines and Therapeutics
- How mRNA Vaccines Induce Immunity
- Advantages of mRNA as a Vaccine Modality
- Plasmid DNA (pDNA) Preparation for mRNA Synthesis
- mRNA In Vitro Transcription and Capping
- mRNA Synthesis Reagents for In Vitro Transcription (IVT)
- Purification and Analysis of mRNA
- Removal of dsRNA During mRNA Synthesis
- Reagents for Purification and Analysis of Synthetic mRNA
The potential for synthetic mRNA to recapitulate the function of mature mRNA has elevated its potential for a spectrum of biopharmaceutical applications. These therapeutics include in vivo delivery of mRNA for protein replacement, stem cell induction, or cancer immunotherapies. Recent significant pharmaceutical uses include novel approaches for vaccines against infectious disease, most notably the mRNA-based vaccines that protect against SARS-CoV-2.
For complete mRNA vaccine products and solutions, visit our Vaccine Development and Manufacturing page.
HOW mRNA VACCINES INDUCE IMMUNITY
Messenger RNA (mRNA)-based vaccines are derived from the central dogma that mRNA encodes for proteins. This straightforward vaccine modality induces an immunological response by delivering the genetic element that codes for all or part of an antigenic protein as a translation-ready molecule. mRNA that encodes the target antigen is taken up by antigen-presenting cells and translated into the target pathogen protein, which in turn induces an immune response. This approach closely mimics the natural process by which viruses infect cells.
ADVANTAGES OF mRNA AS A VACCINE MODALITY
Generally, mRNA vaccines are produced by in vitro synthesis through an enzymatic process. The development and manufacture of mRNA vaccines are relatively simple processes when compared to more traditional modalities, and can be accomplished in a short time period. This attribute makes them suitable for accelerated development and scaleup, which has so far proven critical for emergent public health scenarios. Innovations in mRNA vaccine design and formulation that enhance thermostability so that vaccines would not require cold-chain distribution would reduce production costs and enhance global accessibility.
PLASMID DNA (pDNA) PREPARATION FOR mRNA SYNTHESIS
For mRNA-based vaccine design, in vitro transcription of a plasmid DNA (pDNA) template is typically used to produce functional synthetic mRNA. The plasmid vector usually contains the following elements: an upstream promoter exclusively recognized by T7, SP6 or T3 RNA polymerase, all of which are derived from bacteriophages; 5' UTR, cDNA, 3' UTR, a downstream poly A-tail; and a unique cleavage site downstream of the poly A-tail.

Figure 1.Plasmid generation for in vitro transcription (IVT). Following gene synthesis, cloning of the target DNA uses a plasmid vector (pDNA). The pDNA is expanded in bacterial culture, then purified using nucleic acid purification methods, such as silica-based membranes in spin columns.
mRNA IN VITRO TRANSCRIPTION AND CAPPING
Bacteriophage RNA polymerase is normally used to transcribe linearized plasmid DNA. The pDNA is first linearized with the selected unique restriction site enzyme. After digestion, the linearized pDNA may be purified using methods such as the phenol-chloroform protocol, or the GenElute PCR Clean-Up Kit. For large scale purification, tangential flow filtration (TFF) is often advisable, as it can be easily scaled up. Following linearization, in vitro transcription and capping is performed in a mixed solution of recombinant RNA polymerase (T7, T3 or SP6) and nucleoside triphosphates, plus a cap analog such as CleanCap® Reagent or ARCA (Anti-Reverse Cap Analog). The modified nucleoside such as N1-Methylpseudouridine-5'-Triphosphate (N1-Methylpseudo-UTP, 1-Methylpseudo-UTP) can be used instead of GTP to suppress the innate immune system, and is used in the current mRNA vaccines. The efficiency of capping mRNA with ARCA is 70~80% on average because it competes with GTP, while the yield of mRNA is reduced by about one-fourth compared to standard mRNA synthesis. In comparison, the CleanCap® reagent has been shown to work at 94% capping efficiency without affecting the yield of mRNA production. Alternatively, capping may be achieved by performing the transcription without a cap analog, instead employing the vaccinia virus-encoded capping complex (capping enzyme, 2'-O-Methyltransferase, GTP, and S-adenosyl methionine (SAM)). Capping efficiency will differ depending on the secondary structure of the mRNA of interest. Finally, when the length of the poly A tail of the template pDNA is insufficient (up to 150 bases), it can be extended by use of poly A enzyme.
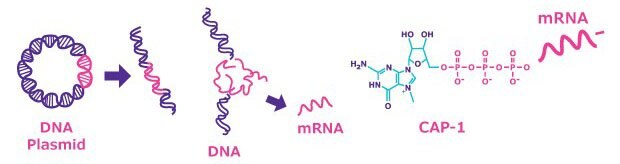
Figure 2.mRNA synthesis is completed by linearization of pDNA, in vitro transcription (IVT) of mRNA using cell-free methods, and capping of the mRNA using cap analog or virus-encoded capping complex.
PURIFICATION AND ANALYSIS OF mRNA
The first step in mRNA purification is to remove the linearized pDNA template using deoxyribonuclease, or DNase. The mRNA is then precipitated with lithium chloride (LiCl), and washed with 75% ethanol. The resulting mRNA pellet can be resuspended with water or resuspension buffer. This precipitation method should, however, be avoided during scale-up, both because it is somewhat difficult to scale, and because the use of hazardous solvents should be avoided under GMP production. The GenElute™ mRNA Miniprep Kit is recommended for smaller scale purification. At larger scales, tangential flow filtration (TFF) can be achieved using Pellicon® Cassettes, and can be scaled in linear fashion.
REMOVAL OF dsRNA DURING mRNA SYNTHESIS
Double-stranded RNA or dsRNA, a transcriptional by-product, is a major concomitant of mRNA synthesis. The dsRNA can stimulate innate immune responses that act to reduce the translation of delivered mRNA. When mRNA is synthesized for vaccine development, dsRNA should therefore be removed. Cellulose-based purification using cellulose fibers can be applied for the removal of dsRNA at various scales.
如要继续阅读,请登录或创建帐户。
暂无帐户?