Metal Organic Frameworks (MOFs)
Josephine Nakhla, Stephen Caskey, Ph.D
Metal-organic frameworks (MOFs) are a class of porous, crystalline materials with a broad range of applications. MOFs are composed of metal ions or clusters, which act as the joints, bound by multidirectional organic ligands, which act as linkers in the network structure. These networks can be 1-D, 2-D, or 3-D extended, periodic structures. The joints and linkers assemble in such a way that regular arrays are formed, resulting in robust (often porous) materials analogous to zeolites. MOFs are the highest reported surface area materials known. Most porous MOFs reported are microporous (pore diameters of less than 2 nm) as defined by IUPAC based on the type of gas sorption isotherm the material displays; however, there have been a limited number of examples of demonstrated mesoporous (pore diameters of 2-50 nm) MOF materials. Besides much greater internal surface areas, MOFs offer significant advantages over zeolites in the prospect of predictable alteration of organic units to provide tailored materials for given applications. For example, the length of the organic linker often defines the size of resulting pores of a given material. Furthermore, functionalization of the organic unit can provide predictably functionalized pores.
We are pleased to offer MOFs under the tradename Basolite™. These materials (Figure 1) provide a good selection of different pore shapes and sizes, different metals (Al, Cu, Fe, and Zn) and different organic linkers (BDC, BTC, mIM).
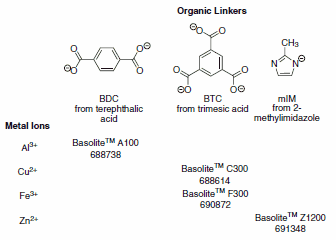
Figure 1.Organic linkers
HKUST-1 (Basolite™ C300)
HKUST-1 is a copper-based MOF that was first reported in 1999 by Williams and co-workers.1 Blue cubic crystals are formed under solvothermal conditions. Under these conditions, CuII paddlewheel dimers form readily to act as square-planar building blocks and are linked by the trimesate trianions that act as trigonal-planar building blocks. These crystals are then exchanged into a low boiling solvent and evacuated under vacuum at elevated temperature to generate a porous material. Prior to evacuation, solvent molecules, generally water, fill the axial coordination positions of the CuII-paddlewheels. Once the coordinating ligands are removed under vacuum, the material becomes sensitive toward re-coordination of the ligand such that irreversible decomposition can occur upon exposure to air/ moisture (Scheme 1). This is generally true of all Cu-based MOFs, but not necessarily for other metals. If the material is handled properly, the Langmuir surface area of HKUST-1 is ca. 2200 m2/g.2 HKUST-1 has been called several different names such as MOF-199 and Cu-BTC; we offer this material as Basolite™ C300 (Product No. 688614).
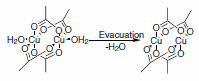
Scheme 1.HKUST-1 (Basolite™ C300)
De Vos has reported the separation of C8-alkylaromatic compounds (p-xylene, m-xylene, and ethylbenzene), which are too close in boiling point to separate by distillation. They investigated HKUST-1 (Basolite™ C300, Product No. 688614), MIL-53(Al) (Basolite™ A100, Product No. 688738) and MIL-47(V). MIL-47, a V-based material, was used in this separation, which is proposed to have been achieved by size selectivity.3-5 The best known example of size selective catalysis using a MOF was reported in JACS in 2008.6 This work was also featured in Chemical & Engineering News.7A Mn-based tetrazole MOF with BET surface area of ca. 2100 m2/g was shown to act as a size-selective Lewis acid catalyst for the cyanosilylation of carbonyls (Scheme 2). While the size-selection aspect of this work is unprecedented for this reaction, it can be catalyzed by several different zeolites as well as HKUST-1 (Basolite™ C300, Product No. 688614).10
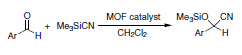
Scheme 2.Cyanosilylation of Carbonyls
ZIFs
MOF materials termed zeolitic imidazolate frameworks (ZIFs) are generated from metal ions and imidazolate anions.9 The bonding angles of the imidazolate are thought to mimic the bonding angles about Si-O bonds found in zeolites; thus, ZIFs and zeolites tend to form closely related structures. ZIFs involve M-N bonds instead of M-O bonds. The thermal stability of ZIFs are reported to be higher than most MOFs, up to ca. 500 ºC, however, organic components are still present, which limit the stability. Some of the most important ZIFs are ZIF-8 (provided under the name Basolite™ Z1200, 691348) and ZIF-69, which is useful for CO2 storage.10 The high thermal stability of ZIFs points to the potential for application as solid supports for catalysis. Several MOFs have already been examined as solid supports, analogous to alumina, silica, or activated carbon, for heterogeneous catalysts to improve surface areas and enhance recyclability. Férey and co-workers recently reported the preparation of Pd-impregrenated MIL- 101, a Cr-based MOF, that showed good activity and recyclability for the Heck reaction of iodobenzene with acrylic acid (Scheme 3).11
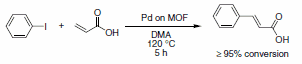
Scheme 3.ZIFs
References
如要继续阅读,请登录或创建帐户。
暂无帐户?