N-Heterocyclic Carbene-Copper Complexes
The electronic and steric modularity associated with the resulting complexes have made N-heterocyclic carbene ligands (NHCs) candidates when designing new metal complexes for catalysis. Nolan and co-workers are among the pioneers in the use of NHC ligands for catalysis, and they have reported the use of Cu-NHCs for a variety of catalytic transformations. Conjugate reduction of a,ß-unsaturated ketones and esters, the hydrosilylation of ketones, the cyclopropanation of terminal alkenes, as well as olefinations, carbene transfer reactions, aziridination of olefins, and methylenation of aldehydes (Scheme 1) are among some examples of the uses of Cu-NHC complexes (specifically (IPr)CuCl) in modern catalysis. Finally, these catalysts are air- and moisture-stable, and they can be used as precursors to synthesize more air-sensitive complexes.1
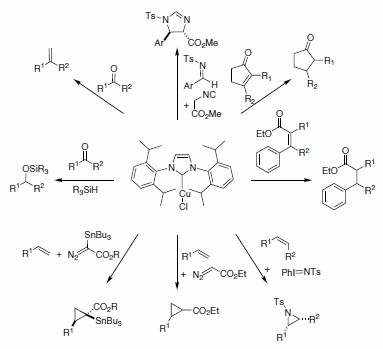
Scheme 1.
Nolan and co-workers have described the use of the Cu-Imes carbene complex 5 in the olefination of aldehydes (and ketones) in the presence of PPh3, i-PrOH, and TMSCHN2. While similar olefinations have been reported using Wilkinson’s catalyst, the Cu-alternative offers a more economical solution. The functional group compatibility is quite good, allowing for the formation of functionalized aliphatic olefins, dienes, and styrenes containing nitro (notoriously deleterious to these types of reactions), trifluoromethyl, amino, and ester functionalities as well as for heteroaromatic olefins substituted with pyridine, pyrrole, and indole derivatives (Scheme 2). The isopropyl-derived carbene complex 6 was also demonstrated to be useful in these types of reactions under slightly modified reaction conditions (Scheme 3).2
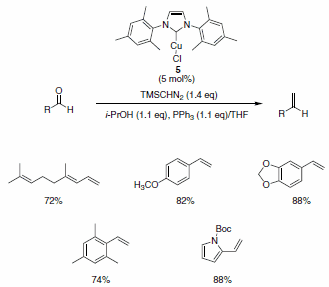
Scheme 2.
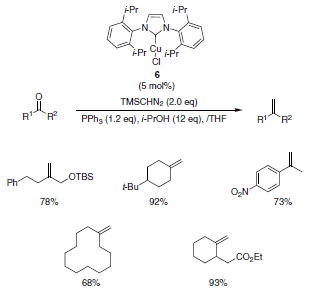
Scheme 3.
References
如要继续阅读,请登录或创建帐户。
暂无帐户?