Of the thousands of chiral ligands used in asymmetric synthesis a relatively large number exhibit C2-symmetry. More recently, non-symmetrical modular P,N-ligands have been introduced independently by Pfaltz, Helmchen, and Williams and applied successfully in various metal-catalyzed reactions.1 These phosphinooxazolines (PHOX) are a highly versatile class of ligands.4
PHOX ligand (43158) was successfully used in Pd-catalyzed asymmetric allylic alkylation reactions. Racemic allyl acetates were transformed into their Pd allyl complex, which is subsequently attacked by a nucleophile to give the corresponding addition adduct (Scheme 1).1-3
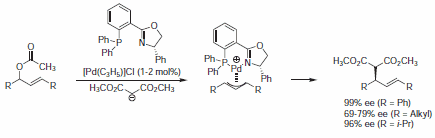
Scheme 1.
Phosphinooxazoline (688495) can induce very high enantioselectivities in the Heck reaction.5-6 In contrast to analogous reactions with diphosphine-Pd catalysts, virtually no C=C double bond migration is observed in the primary product. Hence, particularly in cases where double bond migration leads to undesired products or mixtures of isomers, phosphinooxazolines are the ligands of choice (Scheme 2).
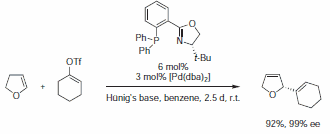
Scheme 2.
Cationic iridium-phosphinooxazoline complexes have proved to be effective catalysts for the enantioselective hydrogenation of imines and trisubstituted olefins (Scheme 3).7 In a systematic study, PHOX ligand with a bis(o-tolyl)phosphanyl moiety gave highest enantioselectivities (up to 97% ee).
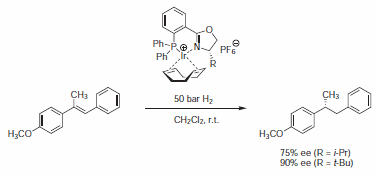
Scheme 3.
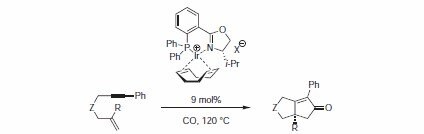
The cationic iridium complex with PHOX ligand (91716) is an efficient catalyst for the enantioselective intramolecular Pauson-Khand reaction.8 Under optimized conditions, enantioselectivities of >90% ee were obtained with 9 mol% of catalyst (Table 1). The anion proved to be important, as it had a significant influence on the enantioselectivity and yield. Best results obtained with relatively small, weakly coordinating anions such as tetrafluoroborate and hexafluorophosphate, but also triflate.
Recently, Rovis et al. used PHOX ligand (688495) in a rhodium(I)-catalyzed enantioselective desymmetrization reaction of meso-3,5‑dimethyl glutaric anhydride, providing rapid access to substituted syn-deoxypolypropionate fragments in a single transformation (Scheme 4).9
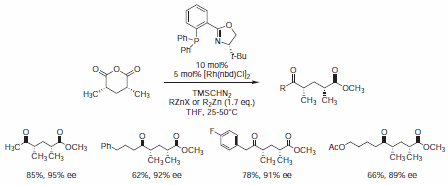
Scheme 4.
References
如要继续阅读,请登录或创建帐户。
暂无帐户?