PLTMax® Human Platelet Lysate (hPL): A Superior Serum-Free Media Supplement for Robust Mesenchymal Stem Cell Expansion
Aletta Schnitzler, Manjula Aysola, Anjali Verma, Tristan Lawson, Susan Rigby, Megan Pease, Sandhya Punreddy, Julie Murrell, Martha Rook
Stem Cell Bioprocessing Group
Introduction
Mesenchymal stem cells (MSCs) have the capacity for multi-lineage differentiation, giving rise to a variety of mesenchymal phenotypes such as osteoblasts (bone), adipocytes (fat), and chondrocytes (cartilage). Stem cell therapy holds immense promise of delivering the next generation of future medical breakthroughs. In this respect, multipotent progenitor cells, such as hMSCs, have attracted high clinical interest because of their ability to differentiate into various cell types and their immunoregulatory properties. Together, these features enable the allogeneic use of hMSCs and thus make them an attractive target for commercial therapeutic development.
The composition of a cell culture media used for hMSCs expansion is of significant concern for development of allogeneic cell therapy products. To date, nearly all such products in clinical trials require added serum in their growth media for expansion, most prominently fetal bovine serum (FBS). This poses an important risk because current good manufacturing practice (CGMP)–quality FBS is of limited availability; a single commercially successful cell therapy product could deplete global FBS supplies. The risk of unknown infectious reagents in FBS is also a significant concern. And some studies of it have demonstrated a risk to patients of immunogenic reactions to xenogenic proteins, which could be transmitted to hMSCs from FBS during culture. Thus, alternatives to FBS are under investigation.
PLTMax® Human Platelet Lysate is a growth factor rich supplement that is a superior alternative to fetal bovine serum (FBS) for human mesenchymal stem cell (MSC) expansion. PLTMax® Human Platelet Lysate has been used to effectively grow mesenchymal stem cells derived from species including human, mouse, rabbit and porcine and has been used in numerous phase I clinical trials worldwide.
Here we investigated whether PLTMax® hPL could be used to expand hMSCs on both normal tissue culture flasks and on microcarriers in bioreactor cultures without affecting the cells’ identity and functionality. We identify PLTMax® hPL as an alternative to FBS for mesenchymal stem cell cultures.
Methods
Human MSC Expansion in Human Platelet Lysate
Human mesenchymal stem cells were derived from bone marrow (SCC034) and cultured as described elsewhere. The culture medium consisted of alpha-MEM (M8042) from supplemented with either 10% MSC-qualified FBS or with 5% PLTMax® supplement (SCM141), 2 mM l-glutamine (A8185), 1× EmbryoMax® penicillin– streptomycin solution (TMS-AB2), 8 ng/mL human recombinant basic fibroblast growth factor (F0291), and 2 U/mL heparin (H3149). Collagen Type I coated microcarriers (CLS3786) were chosen for hMCs expansion in spinner flasks (CLS3561) or Mobius® CellReady single-use bioreactors.
Human MSC Differentiation
MSCs were treated with Accutase (A6964) and grown in either PLTMax® or FBS-containing media off their microcarriers and plated them onto 24-well tissue culture plates at a seeding density of 2.1 × 104 cells/cm2 for inducing adipogenesis and a density of 4.2 × 103 cells/cm2 for inducing osteogenesis, which is described elsewhere.
FACS Mesenchymal Stem Cell Marker Analysis
We resuspended the cell pellets in alpha-MEM with 2% BSA (A9576) to bring the cell concentration to 5 × 105 cells/mL. Our detailed protocol is described elsewhere. Briefly, cells were read on a Guava®easyCyte 8HT flow cytometry system and raw data imported into the FlowJo® program (Treestar) for analysis.
Results
A) PLTMax® hPL B) FBS


Figure 1. Phase bright images of P4 bone-marrow mesenchymal stem cells grown in media containing 5% PLTMax® or 10% FBS. The MSCs in both conditions have the typical fibroblastic-like spindle morphology associated with undifferentiated multipotent MSCs.

Figure 2. PLTMax® hPL supports superior expansion of hMSCs vs. DMEM + FBS in tissue culture and spinner flasks. A) Growth of hMSCs cultured in αMEM supplemented with 5 % PLTMax® hPL or 10 % FBS as compared to DMEM + 10 % FBS over 4 passages in T150 flasks, or B) in duplicate 125 mL spinner flasks or 7 days, demonstrating enhanced growth in αMEM + PLTMax® hPL.

Figure 3.Equivalent biomarker expression and differentiation potential in hMSCs expanded with PLTMax®(hPL) vs. FBS in Mobius® bioreactors. A) hMSCs derived from two adult bone marrow donations (hMSC1 or hMSC3) were expanded in Mobius® CellReady 3L bioreactors in DMEM+5 % PLTMax® or DMEM+10 %FBS. B) hMSC1 cells were analyzed on day 14 by flow cytometry (bar graph) or RT-PCR (inset). Equivalent surface marker levels were detected on hMSCs expanded with PLTMax® in comparison to FBS. Of the 46 hMSC-relevant genes examined, 96 % had high correlation between the two conditions. C) Cells harvested on day 14 were positive for the adipocyte stain Oil Red and for the osteocyte stain Alizarin Red, following the respective differentiation protocols.
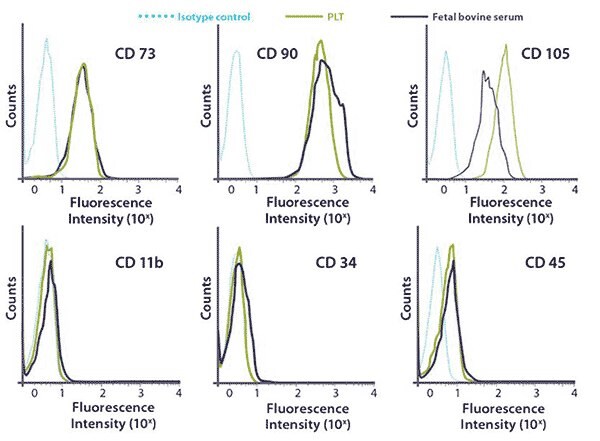
Figure 4.FACS surface marker analysis of hMSCs cultured in PLTMax® vs. FBS in bioreactor cultures. hMSCs cultured in 5% PLTMax® or 10% fetal bovine serum (FBS) media conditions retained their stem cell identity with cell-surface antigen expression positive: CD73, CD90, and CD105 negative: CD11b, CD34 and CD45.
Conclusions
Our data demonstrates the utility of PLTMax® suman platelet lysate (hPL) for both small scale and large-scale expansion of hMSCs in microcarrier-based cultures. We found that hMSCs expanded in hPL maintain their MSC identity and potency similar to previous observations using flatbed cultures. Thus, hPL offers a viable alternative to FBS as a serum supplement for large-scale microcarrier-based hMSC cultures. With recent cell therapy successes, process development for large-scale manufacturing of cells is of increasing interest. So the combination of hPL with microcarrier-based cultures will be an attractive opportunity.
如要继续阅读,请登录或创建帐户。
暂无帐户?