5 Reasons Cancer Researchers Adopt 3D Cell Culture: A Review of Recent Literature
Summary
Introduction
Growing Cells in 3D alters proliferation and cell morphology
Growing cells in 3D reveals a more realistic drug response
Growing cells in 3D captures phenotypic heterogeneity
Growing cells in 3D changes gene expression and cell behavior
Growing cells in 3D mimics the tumor microenvironment
Conclusions
References
Summary
Numerous cancer model systems are available to investigate disease mechanisms and to screen therapies. While all of the models have contributed critical information about cancer biology, current tools have significant problems. Roughly 90% of promising preclinical drugs, in all therapeutic classes, fail to result in efficacious human treatments, thereby wasting vast amounts of time and money and, ultimately, delaying the discovery of successful interventions (van der Worp et al., 2010). In the most simplistic view, two-dimensional (2D) tissue culture models lack realistic complexity, while animal models are expensive, time consuming, and too frequently fail to reflect human tumor biology (Aggarwal et al., 2009; Hait, 2010; van der Worp et al., 2010). This white paper presents five alterations in cellular physiology that suggest why three-dimensional (3D) cultures of cancer cells are a scientifically-rigorous method to generate new drug candidates before moving to expensive and time-consuming animal models. Adding 3D culture to your laboratory’s repertoire can speed discovery and save money when developing cancer models for preclinical screening and testing, as well as for new therapy research and development.

Figure 1.Epithelial tumor and surrounding environment, a complex biology to model. Image from Kimlin et al., 2013
Introduction
Preclinical cancer drug research has the worst success rate of any therapeutic area with a paltry 5% of candidate compounds passing phase III clinical trials for safety and efficacy (Hutchinson and Kirk, 2011). Researchers have devised a vast array of model systems to study the complex components of tumors (Figure 1) and their treatment, each with unique advantages and disadvantages. All models have two key requirements. The first is the ability to monitor cell number and viability since a key characteristic of cancer is uncontrolled cell proliferation sustained by multiple mechanisms. The second is the capacity to study migration and invasion of cancerous cells into surrounding tissues, as metastasis not only damages surrounding tissue, but also complicates treatment. Complex and desirable, yet challenging-to-model features include angiogenesis stimulation and immune system evasion, both of which contribute to long-term tumor survival. The best preclinical model would be relatively inexpensive, amenable to high-throughput screening, and most importantly, reflect human-tumor biology as closely as possible.
The most simplistic cancer models are cell lines derived from human and animal tumors grown as flat monolayers submerged in media. In this configuration, cells are adhering to an artificial plastic or glass substrate and in contact with other cells only at their periphery. Oxygen, nutrient, or waste gradients are not present; the environment is non-physiologically uniform. Coating dishes with extracellular matrix (ECM) can restore a more natural basal adhesion; however, unless the cells are allowed to pile on top of one another they are still being forced into a monolayer morphology, which is not natural for all cell types. Establishing co-cultures in a flat dish can increase natural intercellular contact and communication, but the 2D surface still inhibits the ability for cells to form a multidimensional structure. Cells grown in flat layers on plastic surfaces simply do not reflect the multicellular microenvironment found in the body. There is no doubt that immortalized tumor lines grown in 2D culture have contributed tremendous amounts of knowledge about the mechanism of cancer, but with a 95% drug failure rate, (Hutchinson and Kirk, 2011) they have proven a poor drug development model.
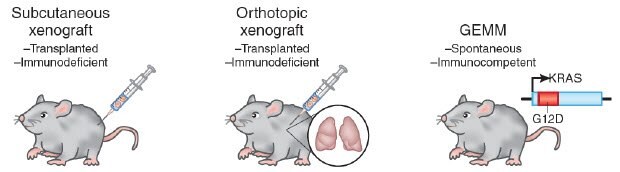
Figure 2.Rodent cancer models. Image adapted from Francia and Kerbel, 2010
Animal models have been used as living incubators in which human tumors have been grown, thereby interacting with a whole organism, albeit frequently an immunodeficient one. Cancer is established in rodent models by either surgically implanting tumor cells or creating geneticallyengineered animals that spontaneously develop human-like tumors in response to experimental modification of gene expression (Figure 2).
At first glance, this seems an inherently desirable system in which to mimic human disease. Despite extensive use for screening, these expensive animal models have failed to develop into therapies that translate to improved outcomes for human disease (Aggarwal et al., 2009; Hait, 2010; van der Worp, et al., 2010). In addition, there are many forms of deadly cancer that currently lack a qualified animal model including brain, kidney, and skin (Steele and Lubet, 2010). The reasons for animal model failures are not completely understood. Determining why will ultimately lead to increased understanding of the disease process, but at present theses models are clearly not generating therapies with a high rate of return on investment.
Scientists have been developing 3D tissue culture models to bridge the gap between in vitro experiments used for discovery and screening and in vivo experiments used for efficacy and safety assessment before proceeding to clinical trials. It is logical to use 3D models with customized microenvironments to dissect cancer biology and establish therapeutic screens, and the evidence that they are a superior system to 2D and early-stage animal testing is rapidly emerging. The purpose of this white paper is to briefly review recent 3D cell culture publications that exemplify the scientific merit of the 3D technique for understanding cancer biology.
Growing Cells in 3D alters proliferation and cell morphology
When cancer cells are grown in a flat monolayer, they proliferate at a relatively uniform rate across the surface. However, growing the same cells in 3D induces zones of differential proliferation (Figure 3) with cell division occurring more rapidly on the outside of the spheroid (Lin and Chang, 2008). Generally this results in a mild reduction in the rate of proliferation for some, but not all, cancer cell lines examined (Chitcholtan, et al., 2013; Longati et al., 2013; Myungjin Lee et al., 2013; Zschenker et al., 2012). The suggested mechanisms for changes in proliferation rate include oxygen/nutrient/growth factor gradients across the spheroid (Lin and Chang 2008), ECM-dependent changes in intracellular signaling, (Kim et al., 2011, Tibbitt and Anseth, 2012), and stromal cell influence (McMillin et al., 2013).
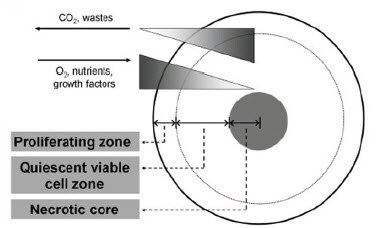
Figure 3.Growth of cells in spheroids results in differential zones of proliferation due to oxygen, nutrient, and waste gradients. Figure from Lin and Chang, 2008.
Growth of cells on a 2D surface results in correspondingly flat cells through simple geometry; there is only one surface on which to adhere. Forcing one side to adhere to a substrate with no opportunity for cellular contact on the opposite side results in default apical-basal polarity and changes in cell shape that ultimately modify cellular function (Baker and Chen, 2012). Merely growing tumor cell lines in 3D, even ovarian epithelial cells with default apical-basal polarity (Figure 4), induces histological morphology reminiscent of the tumor type from which they were derived (Myungjin Lee et al., 2013). When the same ovarian epithelia lines were grown in 2D there was no such histological differentiation. Culture of selected prostate and breast cancer cell lines encapsulated in Matrigel™ revealed either spheroidal or stellate cellular morphology indicative of differences in invasive behavior (Hama et al., 2010). However, similar experiments with endometrial and colon cancer lines failed to generate morphology that was predictive of invasive behaviour (Chitcholtan, et al., 2013; Luca et al., 2013). The variable responses to 3D culture in Matrigel highlight innate variations in malignancies from diverse organs as well as how the microenvironment influences cells to produce clinically-relevant observations. Thus, 3D culture can be used to study tumor morphology and understand differences between lines from tumors of similar and dissimilar organs and tissues.
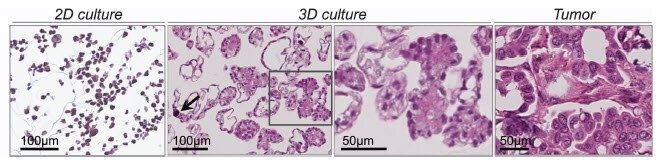
Figure 4.OAW42 epithelial ovarian cancer cells grown in 2D and 3D, the later of which resembles the well-differentiated (Grade 1) serous ovarian tumor shown on the far right. The 3D culture even contains a psammoma body (arrow), a calcified circular structure found in some tumors. Image adapted from (Myungjin Lee et al., 2013).
Growing cells in 3D reveals a more realistic drug response
Growing cells as 3D spheroids can increase resistance to chemotherapy when compared to the same cells grown in monolayers. The most straightforward explanation for enhanced resistance is that the cells on the inside of the spheroid are protected from drug penetration by the cells on the outside of the spheroid (Perche and Torchilin, 2012). Another mechanism has been suggested related to the differential zones of proliferation described above (Chitcholtan et al., 2013). Some chemotherapeutics, like cisplatin, work by inducing DNA damage in proliferating cells severe enough to induce apoptosis. The quiescent population sequestered on the inside of the spheroid will remain protected from drugs like cisplatin, then be free to re-enter the cell cycle once the outer cells are dead. If therapy is halted too early, these “protected” cells may be able to recapitulate a tumor that was shrunk but not completed killed. The mechanisms of drug resistance are likely to be more complicated than just failure to fully penetrate a tumor in vivo and reflected in vitro by spheroid biology. When leukemic cell lines were co-cultured with bone marrow mesenchymal stem cells in either a 2D or 3D system (Figure 5), the 3D culture provided chemoprotection from doxorubicin compared to the 2D culture, despite experiments illustrating that both models allowed complete penetration of the drug (Aljitawi et al., 2013).
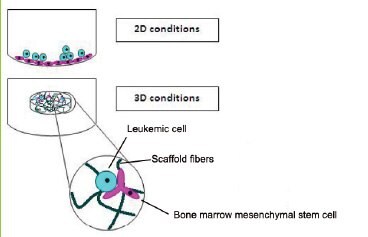
Figure 5.Leukemic 3D cultures were more resistant to doxorubicin than 2D cultures despite equal penetrance of drug into both models. Image from (Aljitawi et al., 2013).
In addition, pancreatic cancer cell lines as well as freshly isolated pancreatic cells from a pancreatic cancer mouse model demonstrated increased expression of several drug resistance genes and microRNAs leading to increased drug resistance in 3D culture as compared to 2D culture (Longati et al., 2013). These results demonstrate that 3D culture recapitulate several mechanisms of drug resistance found in tumors in vivo, offering the opportunity to dissect the mechanisms and test multidrug therapy regimens in vitro before proceeding to animal models and ultimately clinical trials.
Growing cells in 3D captures phenotypic heterogeneity
Heterogeneity occurs across a population of cells within the same tumor as marked by changes in proliferation rate, gene expression, and differentiation resulting in morphological and functional changes (Marjanovic et al., 2013). Wide varieties of phenotypic behavior make targeting drugs to kill the entire tumor challenging since the physiologies can be so divergent. Two theories exist to explain these differences. The clonal selection theory postulates that phenotypic and genotypic heterogeneity is brought about by genetic instability leading to mutations, and perhaps epigenetic changes followed by selection due to competition between cells of an individual tumor (Kreso et al., 2013; Marusyk and Polyak, 2013). Selection pressure could be due to the stress of nutrient deprivation, from tumor growth, or drug treatment. Since tumor spheroids have oxygen and nutrient gradients, 3D models could be established to test these theories with greater ease than serially-passaged mouse xenograft tumors. In fact, a 3D model of ovarian epithelial cancer initiation and progession has been created to study these exact types of genetic changes (Lawrenson et al., 2011).

Figure 6.Rare purported cancer stem cells survive (CSCs) traditional chemotherapeutic treatments and can recapitulate the entire tumor including a new subset of CSCs. Image adapted from Vermeulen et al., 2012.
A second controversial theory hypothesizes that cancer stem cells (CSCs) exist that either seek out a niche created within the tumor microenvironment or are created by signals from a niche itself (Borovski et al., 2011). A miniscule number of cells of an in vitro spheroid or an in vivo tumor exhibit characteristics of “stem cell-like” characteristics including a slowed proliferation rate, selfrenewal and an undifferentiated phenotype that can undergo multilineage differentiation (Schiavoni et al., 2013). CSCs, which can reconstitute tumors following drug treatment (Figure 6), have been described in both 3D spheroid models and in patients undergoing chemotherapy (Chitcholtan et al., 2012). Increased expression of “stemness-related genes” (Busse et al., 2013) was observed when comparing solid tumor cell lines grown as 3D spheroids to monolayers. While clearly not definitive proof of the presence of CSCs, these types of results illustrate the tendency for the in vitro 3D microenvironment to create a “stem cell-like” population similar to the in vivo population believed to be responsible for tumor drug resistance. For arduous to treat and therefore deadly cancers, like glioblastoma, characterizing and reproducing the microenvironment responsible for initiation and survival of CSCs in vitro may be the quickest pathway to potential treatment (Filatova et al., 2013).
Growing cells in 3D changes gene expression and cell behavior
Cells grown in 3D cultures generate divergent gene expression patterns when compared to the exact same cells grown as a monolayer (Myungjin Lee et al., 2013; Luca et al., 2013). Differential extracellular interactions with ECM (Figure 7) and stromal cells in 3D or 3D co-culture models change intracellular signal transduction, culminating in activation of a unique set of transcription factors in 2D versus 3D models (Bellis et al., 2012). Since transcription factors are ultimately responsible for gene expression, changes in cell contact directly results in changes in gene expression. As discussed above, these gene expression changes drive changes in morphology, proliferation rates, and drug resistance, all of which promote a more representative cancer tissue. In turn, these changes in tumor gene expression can alter intercellular feedback to other cells within the culture by modifying the secretion of ECM or signaling factors (Longati et al.,2013).
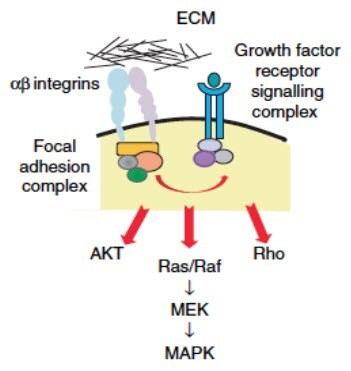
Figure 7.One hypothesis for how ECM controls intracellular signaling is through the binding of different subtypes of transmembrane integrin proteins. Integrins then interact with various intracellular adaptor proteins to form a focal adhesion complex that regulates signaling pathways downstream of growth factor receptors. Image adapted from Kim et al, 2011.
The culmination of these genetic changes has impacts on cell behaviors such as cell migration and differentiation that are inexorably linked to the severity of tumor progression. Typically cell migration has been interrogated in Boyden chamber systems or monolayer scratch assays, but concerted effort is being put forth to reimagine these assays in the more sophisticated 3D environments because the results are more likely to be clinically relevant (Burgstaller et al., 2012). The differences in cell behavior between normal somatic cells and tumor cells grown in 3D can be so pronounced that there is potential to distinguish normal from transformed cells just by growing them in 3D (Fassert et al., 2013). When normal human bronchial epithelial cells are grown in 2D cultures they proliferate until they form a confluent monolayer, but in 3D Matrigel cultures, they differentiate into a characteristic lumen-containing glandular structure, termed an acinus (Wu at al., 2011). When several different epithelial lung cancer lines were grown in 3D Matrigel cultures they formed more spheroids per cell number seeded that failed to differentiate and form a lumen, instead simply increasing in size. The potential of 3D cultures in understanding cancer and perhaps even someday for rapid diagnostic screens are just beginning to be realized.
Growing cells in 3D mimics the tumor microenvironment
As illustrated by all the examples above, cells being grown in 3D cultures much more closely resemble the cells growing within living tumors. One of the most exciting facets of creating in vitro3D cancer models is the ability to experimentally manipulate each component of the tumor microenvironment to try and understand the drivers of cancer as a disease state. Take for example the groundbreaking work of Mina Bissell, whose lab was among the first to recognize that when normal mammary epithelial cells were grown in monolayers they divided exponentially through several passages.
However, when mammary epithelial cells were grown in 3D Matrigel culture, they responded to microenvironmental signals by reducing proliferation and differentiating into nearly normal-sized mammary acinar structures (Figure 8) (Petersen et al., 1992; Tibbitt and Anseth, 2012). Cells derived from breast carcinomas were unable to respond to signals within the Matrigel microenvironment, continuing to divide and failing to form a lumen. By extension, this implies that cancer is a disease of not only the tumor cell but also the surrounding microenvironment. This has important implications for cell lines that have been established and propagated as monolayers. There is no doubt that longterm passage of cells as monolayers could result in loss of the ability to respond to external signals. This could partially explain why cancer cell lines, when returned to a 3D environment, have an incomplete restoration of the original cancer phenotype. In fact, establishing robust spheroid cultures from primary patient samples of colon cancer required the maintenance of some cellular contact as clusters to prevent death (Kondo et al., 2011). Establishing monolayers from tumors may select for rare cells that are able to survive in a highly unnatural environment, perhaps not the best material for modeling tumors.
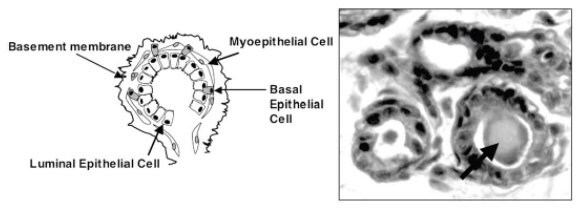
Figure 8.Diagram of a mammary acinus on left and a morphologically similar 3D culture derived MCF-10A acinus on right. The lumens of the in vitro derived acini even contain secreted material on the inside (arrow). Image adapted from Debnath et al., 2003.
No discussion of tumor microenvironment would be complete without mentioning the burgeoning role for cancer-associated fibroblasts (CAFs) in disease progression. The origin of these cells is currently not well understood, but they appear to play a crucial role in the establishment and maintenance of the tumor microenvironment (Allen and Jones 2011; Cirri and Chiarugi, 2011). There is some evidence that signals emanating from the cancer cells within the tumor activate surrounding stromal cells to become CAFs (Figure 9). Normal fibroblasts secrete ECM that comprises the scaffold on which the stromal compartment is arranged. CAFs secrete ECM with an altered protein profile that when compared to normal fibroblasts can have a profound effect on the behavior of tumor cells. In addition, CAFs secrete a variety of growth factors that stimulate proliferation of tumor cells. Based on the growing evidence that CAFs influence tumor proliferation and metastasis, they are emerging as a critical target for combinatorial cancer drug therapies. Including CAFs in a 3D cancer model can increase the understanding of their role in tumor progression and uncover new potential therapies that would remain undiscovered in monolayer models.
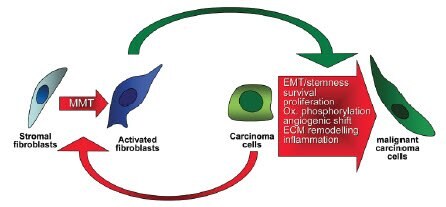
Figure 9.Cancer cells initiate the progression from fibroblast to CAF via a process called MMT (mesenchymal-mesenchymal transition). CAFs then provide a variety of growth factor signals and secrete ECM that promotes the progression, survival, and migration of cancer cells. Image adapted from Cirri and Chiarugi, 2011.
Conclusions
Recapitulating a tumor in vitro requires that all components of the tumor are present, to reproduce typical cell morphology and function, the ECM, proliferating tumor cells, CSC, and stromal cells. The tremendous flexibility of 3D cultures created in Perfecta3D® Hanging Drop Plates allows for the experimental manipulation of all these variables (Figure 10). Each one of these components influences the biology and behavior of the other components, culminating in the functioning tumor model. When cells are removed from a tumor and grown in a monolayer on tissue culture plastic, the loss of these interactions changes their behavior and results in a substandard model for understanding biology or establishing appropriate therapies. This is reflected in the tremendous number of drugs that kill cancerous monolayers, but fail to demonstrate clinical relevance.
Carefully controlling all of the variables discussed above increases the probability that a cancer model is more likely to accurately represent the tumor microenvironment. 3D models can be even more realistic and controllable than human cancer animal xenografts where tumor cells are often transplanted to sites that, while convenient for tumor observation, do not reflect the microenvironment of the original tumor.
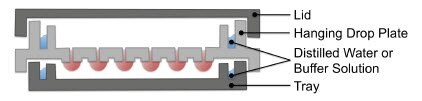
Figure 10.A schematic of the Perfecta3D Hanging Drop Plate. Cultures can be established with a single cell type or as co-cultures, a wide range of starting cell numbers, and with or without addition of exogenous ECM.
Even when the transplantation site is orthotopic, species differences between rodent and human physiology may result in a failure to establish the intricate tumor microenvironment. Despite these limitations, there are certain aspects of efficacy and toxicity that will always require evaluation in animal models prior to human clinical trails. However, preclinical studies that utilize the advantages of 3D culture early on can greatly improve the understanding of cancer biology, eliminate poor drug candidates, and, most promisingly, reveal new more physiologically-relevant targets that might have been missed in 2D screens. Establishing a 3D model system can save time and money by generating more significantly realistic results earlier.
For more information on establishing 3D cultures please visit our 3D Cell Culture Knowledge Center at www.3DBiomatrix.com.
References
如要继续阅读,请登录或创建帐户。
暂无帐户?