Hic Purification Options and Scale Up With Media
Appendix 1, extracted from Hydrophobic Interaction and Reversed Phase Chromatography Principles and Methods (PDF), GE Healthcare, 2014
- Use SOURCE™ media for polishing steps in laboratory or large-scale applications that require highest resolution. Use SOURCE™ media for capture or intermediate purification if a suitable selectivity is not available in a medium of larger particle size.
- Run SOURCE™ columns on systems such as ÄKTAdesign™, FPLC™ System and HPLC. Appendix 3 gives guidance on how to select the most suitable ÄKTAdesign™ system.
SOURCE™ media are based on a hydrophilic matrix made from monodispersed, rigid, polystyrene/divinyl benzene and substituted with hydrophobic ligands: phenyl, ether or isopropyl (Fig 29). The media demonstrate extreme chemical and physical stability. The small particle sizes allow fast binding and dissociation to facilitate high resolution while the uniformity and stability of the particles ensures high flow rates at low back pressure.
The high flow rates that can be used with SOURCE™ media are more likely to be limited by the equipment available rather than the physical properties of the media.
Separation methods can be easily scaled up from prepacked RESOURCE™ columns through to largescale columns such as FineLINE.
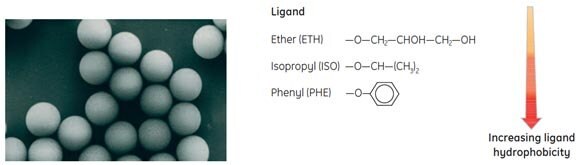
Figure 29. Ligands are coupled to monodispersed SOURCE™ particles via uncharged, chemically stable O-ether linkages.
Purification options
Figure 30. HIC media based on a SOURCE™ matrix are available in prepacked columns and as media packs.
* The nature of the SOURCE™ matrix makes it impossible to define ligand density in the way that is used to compare Sepharose®-based media.
† See Appendix 3 to convert linear flow (cm/hour) to volumetric flow rates (ml/min) and vice versa. Note that final working flow will depend also on factors such as column size and bed height, sample characteristics and loading conditions, the equipment used and the back pressure that the equipment can withstand.
‡ Maximum operating back pressure refers to the pressure above which the medium begins to compress.
It is important to note that the binding capacity of a HIC medium is highly dependent on the properties of the target protein and contaminants, the selectivity of the medium and the binding conditions. Capacity must be determined empirically during media screening and method development.
Use the RESOURCE™ HIC Test Kit, comprising three prepacked RESOURCE™ 1 ml columns (ETH, ISO and PHE), to rapidly screen for the most suitable medium for a specific application. Select the medium that gives the best selectivity, resolution and loading capacity at the lowest salt concentration. Figure 31 shows an example of the differences in selectivity seen during media selection for an IgM purification.

Fig 31.Screening of HIC media for an antibody purification.
- Use prepacked RESOURCE columns for fast media selection, method scouting, group separations or sample concentration.Use SOURCE 15PHE 4.6/100 PE to improve resolution by increasing column length.
- Further optimization may be required. Use optimized conditions as the first step toward scaling up
Select a production column such as FineLINE for larger volumes.
Purification examples
Method optimization
Figure 32 shows examples of runs made while optimizing the intermediate purification step for a recombinant protein, tyrosine phosphatase. The protein was partially purified in an initial capture step, using ion exchange chromatography on Q Sepharose XL. The active fraction was isolated and applied to a SOURCE 15PHE 4.6/100 PE column for intermediate purification by HIC. Results show that increasing the ammonium sulfate concentration used during sample application and increasing the gradient volume used for elution had the most significant impact on resolution. Note that, after the first run, glycerol was included to reduce the strong binding of tyrosine phosphatase and facilitate elution (refer to page 35 for further details on additives used during HIC separations).
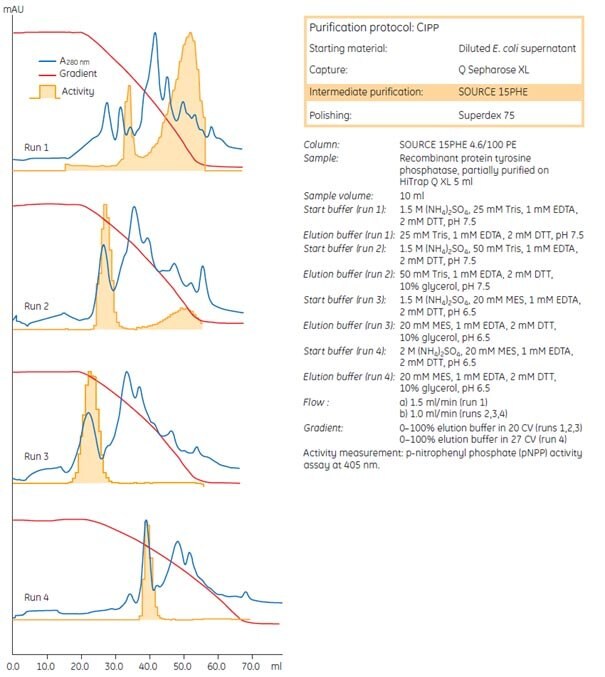
Fig 32.Optimization steps for intermediate purification of a recombinant protein.
Sclaing Up
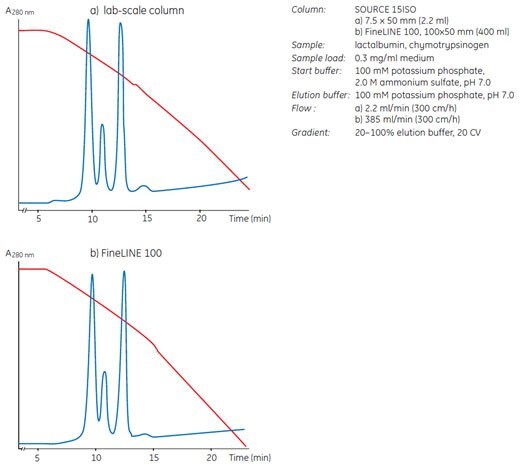
Fig 33. Reproducible results when scaling up on SOURCE™ 15ISO. Separation of a model protein mixture shows a 180-fold scale-up from a laboratory-scale column (a) to a FineLINE 100 production-scale column (b).
Polishing
Figure 34 shows the use of SOURCE™ 15PHE as the final polishing step in a large-scale purification of a recombinant protein, rExotoxin A (PE553D), expressed in the periplasm of rPseudomonas aeruginosa.
Ammonium sulfate (1.0 M) was added to partially purified protein before sample application. The bound exotoxin A was eluted using a linear gradient from 1.0 to 0.55 M ammonium sulfate over 15 column volumes. This step removed the remaining contaminant proteins, as shown by a single peak on reversed phase chromatography (Figure 35).
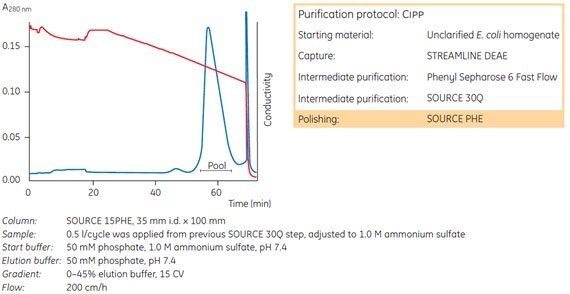
Fig 34. SOURCE™ 15PHE used as the final polishing step in a large-scale purification of a recombinant protein, rExotoxin A (PE553D).
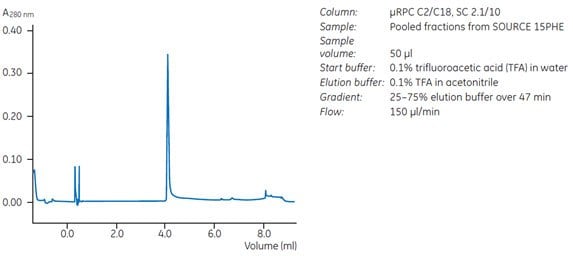
Fig 35. Chromatographic analyses demonstrate purity after polishing step on SOURCE™ 15PHE.
如要继续阅读,请登录或创建帐户。
暂无帐户?