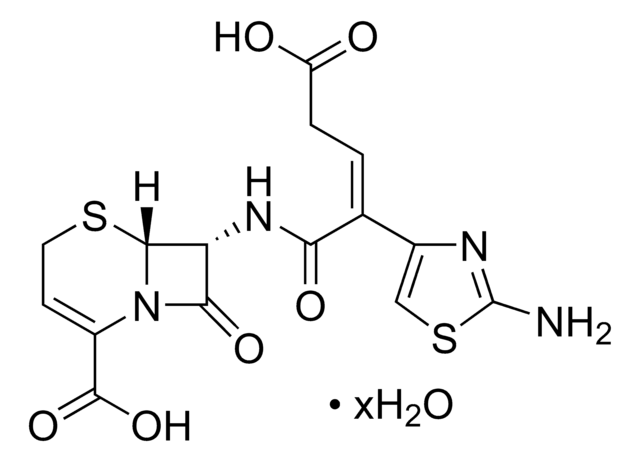
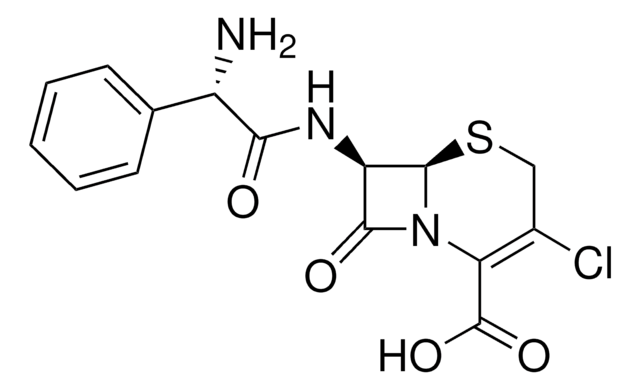
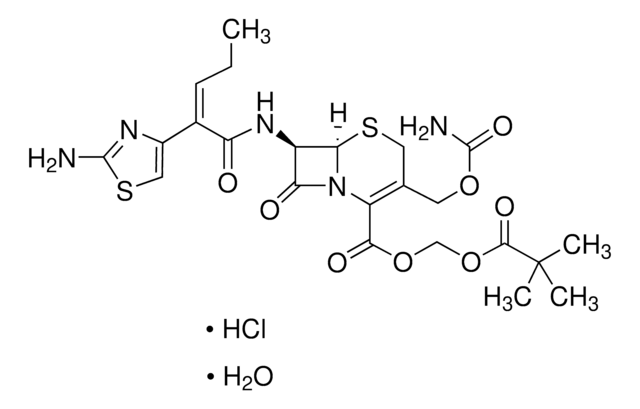
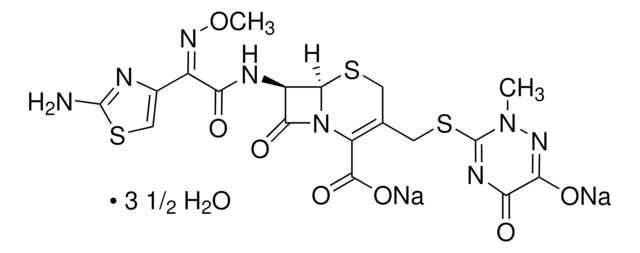
推荐产品
品質等級
化驗
≥97.0% (HPLC)
形狀
powder or crystals
pKa
9.70
mp
170 °C
抗生素活性譜
Gram-negative bacteria
Gram-positive bacteria
作用方式
cell wall synthesis | interferes
儲存溫度
room temp
InChI
1S/C14H13N5O5S2/c1-2-5-3-25-12-8(11(21)19(12)9(5)13(22)23)17-10(20)7(18-24)6-4-26-14(15)16-6/h2,4,8,12,24H,1,3H2,(H2,15,16)(H,17,20)(H,22,23)/b18-7-/t8-,12-/m1/s1
InChI 密鑰
RTXOFQZKPXMALH-GHXIOONMSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
Chemical structure: ß-lactam
應用
An advanced-generation, cephalosporin antibiotic. Used for its excellent and well balanced antibacterial activities against gram-positive and gram-negative bacteria.
Cefdinir was used to study bacterial infections caused by Staphylococcus aureus and Neisseria gonorrhoeae and the horizontal transfer of the ftsI gene in H. influenzae.
生化/生理作用
Cefdinir is an advanced generation amino-2-thiazolyl cephalosporin effective against Gram-positive and Gram-negative bacteria. It acts by disrupting the synthesis of the peptidoglycan layer of bacterial cell walls. It is also known to inhibit human neutrophil myeloperoxidases.
其他說明
Keep container tightly closed in a dry and well-ventilated place. Keep in a dry place.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Janet R Casey et al.
Drugs, 72(15), 1991-1997 (2012-10-09)
10 days of amoxicillin/clavulanic acid high dose and 5 days of cefdinir have been the preferred first- or second-line antibiotics for treatment of children with acute otitis media (AOM) since 2004, as recommended by the American Academy of Pediatrics in
Indi Trehan et al.
The New England journal of medicine, 368(5), 425-435 (2013-02-01)
Severe acute malnutrition contributes to 1 million deaths among children annually. Adding routine antibiotic agents to nutritional therapy may increase recovery rates and decrease mortality among children with severe acute malnutrition treated in the community. In this randomized, double-blind, placebo-controlled
M T Labro et al.
Journal of immunology (Baltimore, Md. : 1950), 152(5), 2447-2455 (1994-03-01)
Cefdinir, a new oral 2-amino-5-thiazolyl cephalosporin, inhibited the luminol-amplified chemiluminescence (LACL) response of human neutrophils stimulated by PMA but not opsonized zymosan, in a concentration-dependent but not time-dependent manner. The LACL response to opsonized zymosan in cytochalasin B-treated neutrophils was
Helio S Sader et al.
Expert review of anti-infective therapy, 5(1), 29-43 (2007-02-03)
Cefdinir is an oral third-generation cephalosporin (also known as an advanced-spectrum or generation cephem) with good in vitro activity against the pathogens responsible for community-acquired respiratory tract infections and uncomplicated skin and skin structure infections. The drug distributes very well
D R Guay
The Pediatric infectious disease journal, 19(12 Suppl), S141-S146 (2001-01-06)
Oral second and third generation cephalosporins are undergoing continuing research and development in the arena of pediatric infectious disease in an attempt to fill voids created by existing agents in the quest for the "ideal" antimicrobial. This paper reviews the
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门