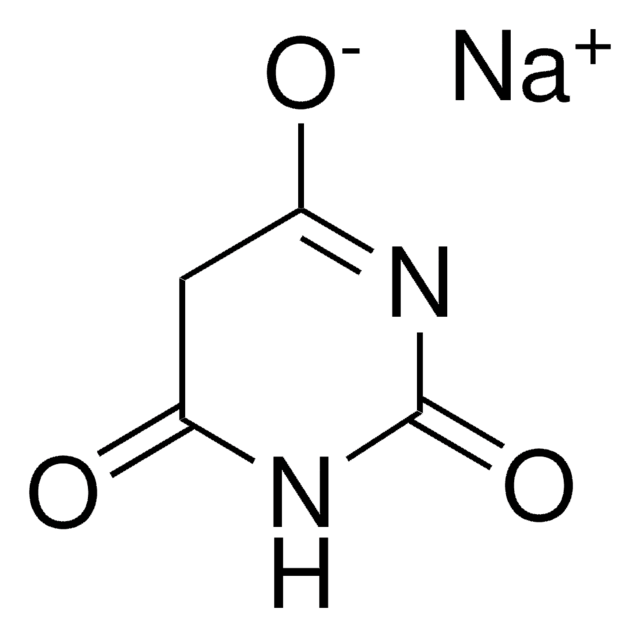
推荐产品
品質等級
化驗
≥99.5% (HPLC)
≥99.5%
形狀
solid
品質
for spectrophotometric det. of cyanide
技術
UV/Vis spectroscopy: suitable
燃燒殘留物
≤0.05%
mp
248-252 °C (dec.) (lit.)
負離子痕跡
chloride (Cl-): ≤50 mg/kg
sulfate (SO42-): ≤500 mg/kg
正離子痕跡
Ca: ≤10 mg/kg
Cd: ≤5 mg/kg
Co: ≤5 mg/kg
Cr: ≤5 mg/kg
Cu: ≤5 mg/kg
Fe: ≤5 mg/kg
Mg: ≤10 mg/kg
Mn: ≤5 mg/kg
Ni: ≤5 mg/kg
Pb: ≤5 mg/kg
Zn: ≤5 mg/kg
SMILES 字串
O=C1CC(=O)NC(=O)N1
InChI
1S/C4H4N2O3/c7-2-1-3(8)6-4(9)5-2/h1H2,(H2,5,6,7,8,9)
InChI 密鑰
HNYOPLTXPVRDBG-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
相关类别
一般說明
巴比妥酸也被称为丙二酸脲或 6-羟基尿嘧啶,它是一种无臭粉末,可溶于水。它具有很高的药理活性,可用来合成加成衍生物,用于新药开发。它基本上作用于中枢神经系统 (CNS) 抑制剂,因此具有从轻度镇静到完全麻醉的广泛范围。
應用
巴比妥酸与芳香醛在微波辐射下,无有机溶剂存在下,在碱性氧化铝上发生 Knoevenagel 缩合反应的效率提高。它也可用于碘的电化学氧化,使用循环伏安法和控制电位库仑法。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°F)
302.0 °F - closed cup
閃點(°C)
150.00 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Electrochemical study of iodide in the presence of barbituric acid. Application to coulometric titration of barbituric acid.
Nematollahi D and Hesari M
Microchemical Journal, Devoted to the Application of Microtechniques in All Branches of Science, 70 (1), 7-11 (2001)
Microwave enhanced knoevenagel condensation of barbituric acid with aromatic aldehydes on basic alumina.
Khalafi NA and Hashemi A.
Iranian Journal of Chemistry and Chemical Engineering, 20 (1), 9-11 (2001)
Bioactive Heterocyclic Compound Classes.
Clemens L and Dinges J.
Pharmaceutics, 24-27 (2012)
Sergio Hidalgo-Figueroa et al.
Chemical biology & drug design, 81(4), 474-483 (2013-01-08)
A small series of thiazolidine-2,4-dione and barbituric acid derivatives 1-4 was prepared using a short synthetic route, and all compounds were characterized by elemental analysis, mass spectrometry, and NMR ((1)H, (13)C) spectroscopy. Their in vitro relative expression of peroxisome proliferator-activated
David C Chiara et al.
The Journal of biological chemistry, 288(27), 19343-19357 (2013-05-17)
GABA type A receptors (GABAAR), the brain's major inhibitory neurotransmitter receptors, are the targets for many general anesthetics, including volatile anesthetics, etomidate, propofol, and barbiturates. How such structurally diverse agents can act similarly as positive allosteric modulators of GABAARs remains
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门