5.09911
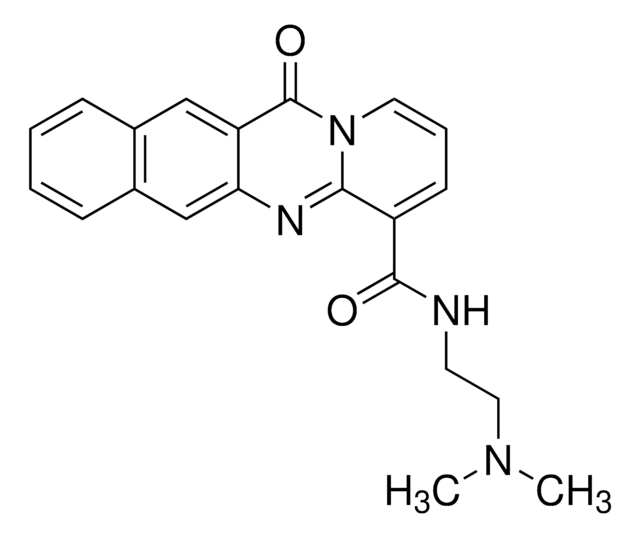
RNA Polymerase I Inhibitor II, BMH-21
别名:
RNA Polymerase I Inhibitor II, BMH-21, BMH21, rRNA Transcription Inhibitor II, N-(2-(Dimethylamino)ethyl)-12-oxo-12H-benzo[g]pyrido[2,1-b]quinazoline-4-carboxamide, N-(2-(Dimethylamino)ethyl)-12-oxo-12H-benzo[g]pyrido[2,1-b]quinazoline-4-carboxamide, BMH21, rRNA Transcription Inhibitor II
登录查看公司和协议定价
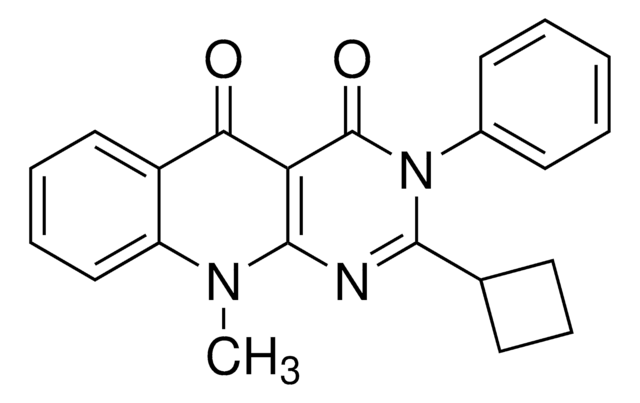
所有图片(1)
About This Item
推荐产品
化驗
≥98% (HPLC)
品質等級
形狀
powder
製造商/商標名
Calbiochem®
儲存條件
OK to freeze
protect from light
顏色
orange-yellow
溶解度
DMSO: 1 mg/mL
5% acetic acid: 25 mg/mL
儲存溫度
2-8°C
一般說明
A cell-permeable, non-toxic, benzopyridoquinazoline-carboxamide compound that blocks the growth and viability of a variety of cancer cell lines (GI50 = 160 nM in NCI-60 cells), but does not significantly affect normal cells (~90-fold therapeutic window). Preferentially binds to the GC-rich DNA and inhibits RNA polymerase I (Pol I) transcription. Although it intercalates with DNA, it does not activate the DNA damage response. Induces a proteasome-dependent destruction of large Pol I catalytic subunit RPA194, independent of TP53 genetic status of cancer cells, leading to disassembly of Pol I holocomplex from the rDNA. However, it does not affect Rpb1, the large subunit of RNA polymerase II and Pol I preinitiation complex proteins. Effectively reduces the growth of A375 and HCT116 xenografts in athymic NCr nu/nu mice (50 mg/kg, i.p, 2 weeks).
Please note that the molecular weight for this compound is batch-specific due to variable water content.
Please note that the molecular weight for this compound is batch-specific due to variable water content.
A cell-permeable, reversible DNA-intercalating benzopyridoquinazoline-carboxamide that preferentially targets GC-rich sequence, notably that of rDNA (162% of average human genome GC content), and effectively inhibits RNA polymerase I- (Pol I) mediated rDNA transcription both in cell-free assays and in cultures (IC50/ICmax = 60 nM/≤1 M against 2-h de novo 47S transcription in A375 cells) without affecting the maturation/processing of 47S into 32S and 18S rRNA. Time-dependent studies in 1 M BMH-21-treated A375 cells reveal fast inhibition of nuclear RNA synthesis (FUrd incorporation) within 15 min, followed by progressive altered localization of nucleolar proteins (starting in <20 min), including Pol I subunit RPA194 capping structure formation, indicative of stalled Pol I complex, and eventual proteasome-mediated RPA194 degradation (>1 h). Although BMH-21 activates p53 independent of DNA damage-sensing ATM pathway signaling in A375 cultures, wt p53 is not a prerequisite for BMH-21 anticancer activity (Av GI50 = 110 nM/wt and 205 nM/mutant among NCI60 cancer panel). Intraperitoneal injection is demonstrated to be efficacious in suppressing A375 (25 & 50 mg/kg/d, 6d/wk) and HCT-116 (50 mg/kg/d, 7 d/wk) tumor growth in mice in vivo. MG-132 (Cat. Nos. 474790, 474787, 474788, and 474791) effectively prevents BMH-21-induced RPA194 degradation without restoring stalled rRNA synthesis.
生化/生理作用
Cell permeable: yes
Primary Target
rDNA
rDNA
Reversible: yes
包裝
Packaged under inert gas
警告
Toxicity: Standard Handling (A)
重構
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 6 months at -20°C.
其他說明
Peltonen, K., et al. 2014. Cancer Cell25, 77.
Peltonen, K., et al. 2010. PLoS One5, e12996.
Peltonen, K., et al. 2010. PLoS One5, e12996.
法律資訊
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门