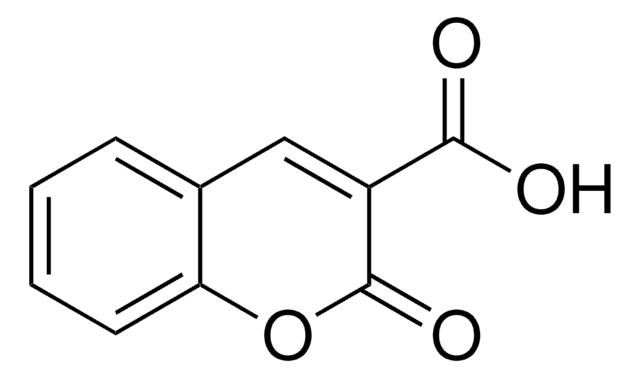
推荐产品
應用
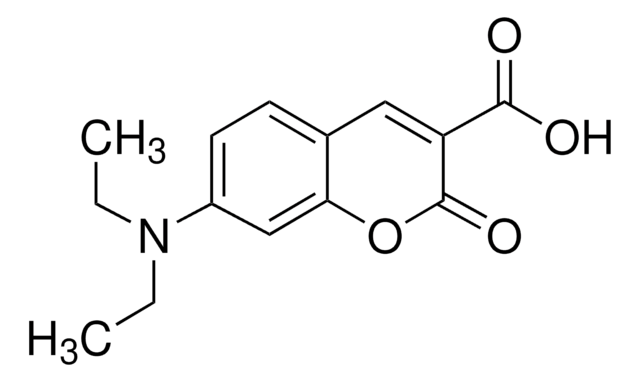
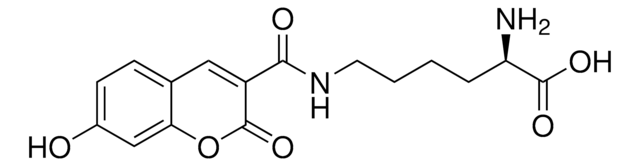
The courmarin-based unnatural amino acid is an effective fluorophore; which has been incoporated into several studies of protein interactions.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Timo Koopmans et al.
Bioorganic & medicinal chemistry, 21(2), 553-559 (2012-12-15)
Incorporation of the unnatural amino acid L-(7-hydroxycoumarin-4-yl)ethylglycine (7-HC) is a powerful and reliable approach for the preparation of fluorescently labeled proteins. The growing popularity of this valuable amino acid prompted us to pursue an improved protocol for its synthetic preparation.
Ishu Saraogi et al.
Journal of the American Chemical Society, 133(38), 14936-14939 (2011-08-30)
As newly synthesized proteins emerge from the ribosome, they interact with a variety of cotranslational cellular machineries that facilitate their proper folding, maturation, and localization. These interactions are essential for proper function of the cell, and the ability to study
Jiangyun Wang et al.
Journal of the American Chemical Society, 128(27), 8738-8739 (2006-07-06)
The fluorescent amino acid l-(7-hydroxycoumarin-4-yl) ethylglycine 1 has been genetically encoded in E. coli in response to the amber TAG codon. Because of its high fluorescence quantum yield, relatively large Stoke's shift, and sensitivity to both pH and polarity, this
Douglas D Young et al.
Bioorganic & medicinal chemistry letters, 21(24), 7502-7504 (2011-11-02)
The site-specific incorporation of unnatural amino acids (UAAs) into proteins in bacteria is made possible by the evolution of aminoacyl-tRNA synthetases that selectively recognize and aminoacylate the amino acid of interest. Recently we have discovered that some of the previously
Shengxi Chen et al.
Journal of the American Chemical Society, 135(35), 12924-12927 (2013-08-15)
Two fluorescent amino acids, including the novel fluorescent species 4-biphenyl-l-phenylalanine (1), have been incorporated at positions 17 and 115 of dihydrofolate reductase (DHFR) to enable a study of conformational changes associated with inhibitor binding. Unlike most studies involving fluorescently labeled
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门