所有图片(3)
About This Item
经验公式(希尔记法):
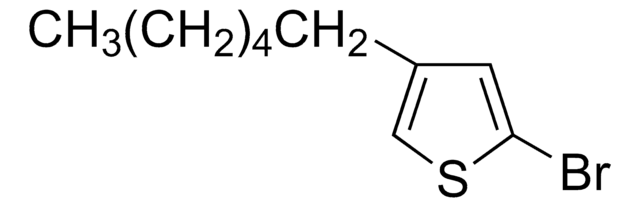
C10H15BrS
CAS号:
分子量:
247.20
MDL號碼:
分類程式碼代碼:
12352103
PubChem物質ID:
NACRES:
NA.23
推荐产品
化驗
97%
形狀
liquid
折射率
n20/D 1.529
密度
1.240 g/mL at 25 °C
SMILES 字串
CCCCCCc1ccsc1Br
InChI
1S/C10H15BrS/c1-2-3-4-5-6-9-7-8-12-10(9)11/h7-8H,2-6H2,1H3
InChI 密鑰
XQJNXCHDODCAJF-UHFFFAOYSA-N
一般說明
2-Bromo-3-hexylthiophene is a monomeric precursor that forms bromo terminate polymers. It is synthesized by the bromination of hexylthiophene.
應用
2-Bromo-3-hexylthiophene is majorly used in the formation of π-conjugated conductive polymers (CPs) for the fabrication of organic field effect transistors (OFETs) and organic photovoltaics (OPVs). The Grignard metathesis (GRIM) polymerization of 2-Bromo-3-hexylthiophene terminates a bromine and a proton at both ends to form conductive poly(3-hexylthiophene) (P3HT).
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral - Aquatic Chronic 4
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
>230.0 °F
閃點(°C)
> 110 °C
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Grignard metathesis (GRIM) polymerization for the synthesis of conjugated block copolymers containing regioregular poly (3-hexylthiophene).
Stefan MC, et al.
Polym. Chem., 3(7), 1693-1701 (2012)
Field-effect transistors based on poly (3-hexylthiophene): Effect of impurities.
Urien M, et al.
Organic Electronics, 8(6), 727-734 (2007)
Synthesis of End-capped Regioregular Poly (3-hexylthiophene) s via Direct Arylation.
Wang Q, et al.
Macromolecular Rapid Communications, 33(14), 1203-1207 (2012)
Synthesis and characterization of poly (3-hexylthiophene)-b-polystyrene for photovoltaic application.
Gu Z, et al.
Polymers, 3(1), 558-570 (2011)
商品
Oligothiophenes are important organic electronic materials which can be produced using synthetic intermediates and Suzuki coupling.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门
![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

![[1,3-双(二苯基膦)丙烷]二氯化镍(II)](/deepweb/assets/sigmaaldrich/product/structures/844/065/af07f787-c6a3-4a6e-a22b-47a933c73978/640/af07f787-c6a3-4a6e-a22b-47a933c73978.png)