所有图片(2)
About This Item
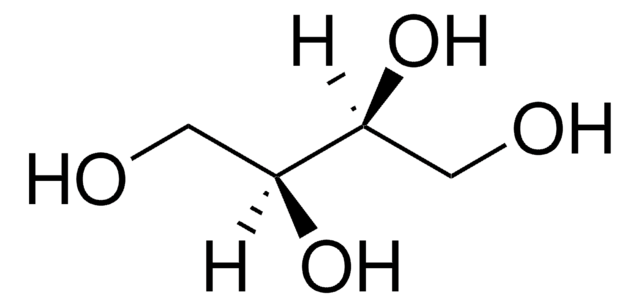
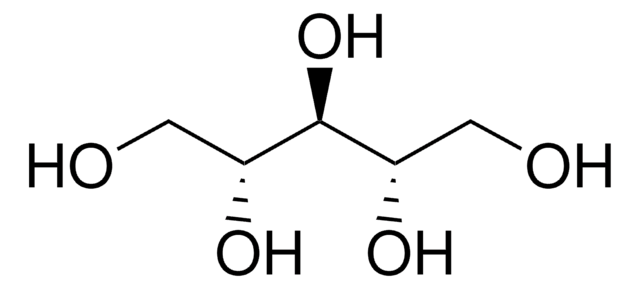
线性分子式:
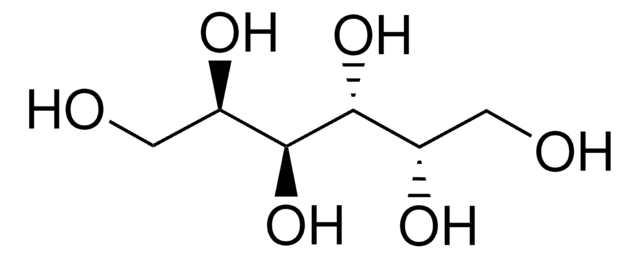
HOCH2[CH(OH)]2CH2OH
CAS号:
分子量:
122.12
Beilstein:
1719752
MDL號碼:
分類程式碼代碼:
12352201
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
99%
形狀
solid
光學活性
[α]20/D −14°, c = 2 in ethanol
mp
88-90 °C (lit.)
SMILES 字串
OC[C@@H](O)[C@H](O)CO
InChI
1S/C4H10O4/c5-1-3(7)4(8)2-6/h3-8H,1-2H2/t3-,4-/m1/s1
InChI 密鑰
UNXHWFMMPAWVPI-QWWZWVQMSA-N
正在寻找类似产品? 访问 产品对比指南
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Jonathan D Silk et al.
Journal of immunology (Baltimore, Md. : 1950), 180(10), 6452-6456 (2008-05-06)
Invariant NKT cells (iNKT cells) recognize CD1d/glycolipid complexes. We demonstrate that the nonglycosidic compound threitolceramide efficiently activates iNKT cells, resulting in dendritic cell (DC) maturation and the priming of Ag-specific T and B cells. Threitolceramide-pulsed DCs are more resistant to
M C Alliegro
Analytical biochemistry, 282(1), 102-106 (2000-06-22)
Dithiothreitol (DTT) is widely used to reduce disulfide bonds in the analysis of protein structure and function. However, thiol-disulfide exchange is not the only mechanism whereby DTT can alter protein function. We observe that DTT diminishes the carbohydrate binding activity
B J Ortwerth et al.
Experimental eye research, 58(6), 665-674 (1994-06-01)
L-Threose is a significant degradation product of ascorbic acid at pH 7.0 in the presence of oxygen. When compared to several other ascorbate-derived degradation products, it had the greatest ability to glycate and crosslink lens proteins in vitro. To determine
Amplification of dynamic chiral crown ether complexes during cyclic acetal formation.
Benzion Fuchs et al.
Angewandte Chemie (International ed. in English), 42(35), 4220-4224 (2003-09-23)
Timothy M Chapman et al.
Journal of the American Chemical Society, 127(2), 506-507 (2005-01-13)
Chlorination-elimination chemistry coupled with three-component Joullié-Ugi reaction and facile deprotection allowed efficient access to an array of polyhydroxylated pyrrolidines through parallel synthesis that may be considered to be a library of imino (aza) sugars (glycomimetics) and/or dihydroxyprolyl peptides (peptidomimetics). The
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门