推荐产品
化驗
99%
形狀
solid
mp
289 °C (dec.) (lit.)
SMILES 字串
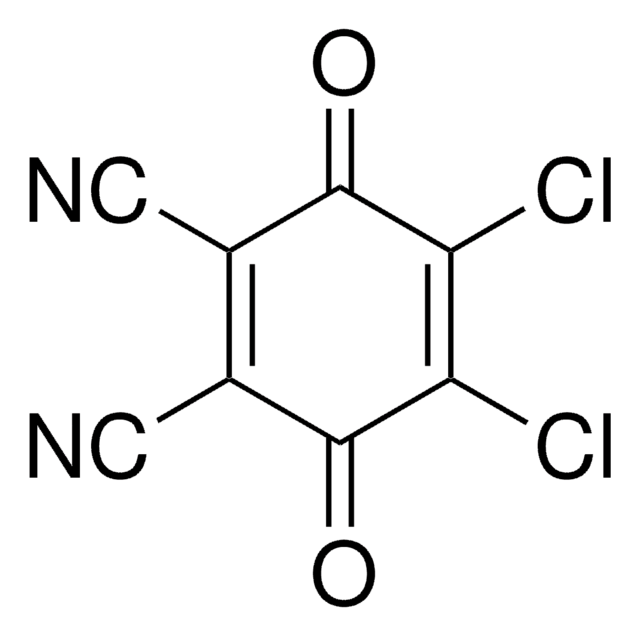
ClC1=C(Cl)C(=O)C(Cl)=C(Cl)C1=O
InChI
1S/C6Cl4O2/c7-1-2(8)6(12)4(10)3(9)5(1)11
InChI 密鑰
UGNWTBMOAKPKBL-UHFFFAOYSA-N
基因資訊
human ... ACHE(43) , BCHE(590) , CES1(1066)
一般說明
Tetrachloro-1,4-benzoquinone (TCBQ) is a quinone compound with four chloride groups. The catalytic activity of quinone groups (benzoquinone) can be controlled by the chloride groups with large electronegativity.
應用
可与二苯乙烯衍生物、α,β-不饱和羰基化合物发生光诱导的环加成反应。荧光猝灭剂。
用于通过氧化环化反应合成二苯并呋喃。
TCBQ can form a nanocomposite with multi-walled carbon nanotubes (MWCNTs) on a graphite electrode for nicotinamide adenine dinucleotide (NADH) oxidation. It can also provide pseudocapacitance and can be used as an electrode material for the development of supercapacitors. It may be used as an organic cathode and act as a redox mediator for the fabrication of lithium ion batteries.
訊號詞
Danger
危險分類
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Skin Irrit. 2 - Skin Sens. 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Electrocatalytic activity of 2, 3, 5, 6-tetrachloro-1, 4-benzoquinone/multi-walled carbon nanotubes immobilized on edge plane pyrolytic graphite electrode for NADH oxidation.
Silva L, et al.
Electrochimica Acta, 53(14), 4706-4714 (2008)
J. Prakt. Chem./Chem.-Ztg., 335, 515-515 (1993)
Tetsuya Takeya et al.
Organic letters, 9(15), 2807-2810 (2007-06-22)
A novel oxidative cyclization of quinone-arenols 5 leading to products 6 with a dibenzofuran-1,4-dione structure, which forms the core of several natural products, has been developed and applied to the synthesis of violet-quinone (4).
Journal of the Chemical Society. Perkin Transactions 1, 571-571 (1994)
An Insoluble Benzoquinone-Based Organic Cathode for Use in Rechargeable Lithium-Ion Batteries.
Luo Z, et al.
Angewandte Chemie (International ed. in English), 56(41), 12561-12565 (2017)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门