MPhos Ligands & Catalysts – Introduction, Advantages, and Applications
Merck has recently developed a series of highly tunable unsymmetrical Ferrocene ligands, MPhos and their corresponding Palladium (Pd) complexes (Figure 1), by incorporating a bulky di(1-adamantyl)phosphino motif on the ferrocene backbone. This new class of ligands and catalysts are very versatile for many types of Csp2-Csp3 (aryl/heteroaryl – alkyl) couplings, i.e. Murahashi−Feringa (Li), Kumada−Corriu (Mg), Negishi (Zn) and Suzuki−Miyaura (B), with broad substrate scope including many “drug-like” molecules, excellent functional group tolerance and branched/linear selectivity.1 The high tunability of the ferrocene back-bone gives the opportunity to fine-tune the ligand/catalyst structure for different kinds of challenging nucleophiles and electrophiles.
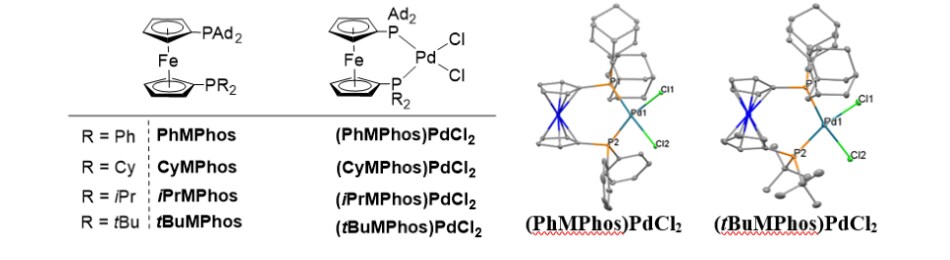
Figure 1.MPhos and its corresponding (MPhos)PdCl2 complexes, and 2) single crystal structures of (PhMPhos)PdCl2 and (tBuMPhos)PdCl2
Applications on Csp2-Csp3 couplings
With relatively low catalyst loading, i.e. 1 mol%, (MPhos)PdCl2 has been demonstrated on the strong ability to effect many kinds of Csp2-Csp3 cross-couplings under mild conditions (examples in Scheme 1). Substrates (Aryl halides, electrophiles) with both electron donating and electron withdrawing, as well as steric hindered and/or heterocyclic aryl halides, all worked very nicely with good to excellent isolated yields. Alkyl nucleophiles, i.e. primary alkyl-, secondary alkyl-, cyclic alkyl-, also gave very good isolated yields and minimum isomerization. Sequential Csp2-Csp3 coupling of the substrates containing both Cl and Br groups has also been examined and proved to be successful, e.g. 5a, 87% and 5b, 91% from 5a, using our (MPhos)PdCl2 catalyst system.
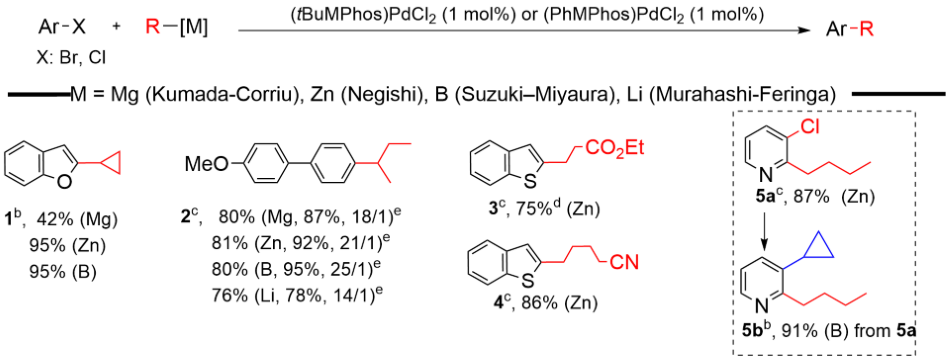
Scheme 1.Examples of Csp2-Csp3 coupling using (MPhos)PdCl2 as the catalyst under Kumada-Corriu (Mg), Negishi (Zn), Suzuki–Miyaura (B) and Murahashi-Feringa (Li) conditions.a
Reaction Conditions: aKumada−Corriu conditions (Mg): Ar-X (0.8 mmol), RMgX (2.0 equiv.), catalyst (1 mol%), THF, room temperature or 50oC. Negishi conditions (Zn): Ar-X (0.8 mmol), RZnX (2.0 equiv.), catalyst (1 mol%), THF, room temperature or 50oC. Suzuki–Miyaura conditions (B): Ar-X (0.8 mmol), RB(OH)2 (2.0 equiv.), catalyst (1 mol%), K3PO4 (3 equiv.), Toluene/H2O (10/1), 100oC. Murahashi-Feringa conditions (Li): Ar-X (0.8 mmol), RLi (1.2 equiv., diluted with Toluene to 0.2 M), catalyst (1 mol%), Toluene, room temperature. Slow addition using syringe pump for 2 hours. bCatalyst: (tBuMPhos)PdCl2. cCatalyst: (PhMPhos)PdCl2. dReaction temperature: 50oC. eGC yield with branch/linear ratio in parathesis. Branch/linear ratio was determined by GC.
Application of Csp2-Csp3 couplings on “drug-like” molecules
Late-stage functionalization on small molecule drugs, i.e. deuterium labelled drugs, especially CD3 based molecules, and drug molecules with cyclopropane fragment, are prominent in pharmaceuticals (Scheme 2). In this regard, Csp2-Csp3 couplings using (MPhos)PdCl2 as the catalyst was applied on grafting various “alkyl fragments” onto the “drug-like” molecules, i.e. “Chemistry Informer Libraries” developed by Merck & Co to mimic “late stage functionalization” for various cross-coupling chemistries of much more complex drug-like molecules, and proved to be very successful (examples in Scheme 3).
Example for Drug (Drug Candidate) Molecules Containing CD3 or Cyclopropyl Fragment
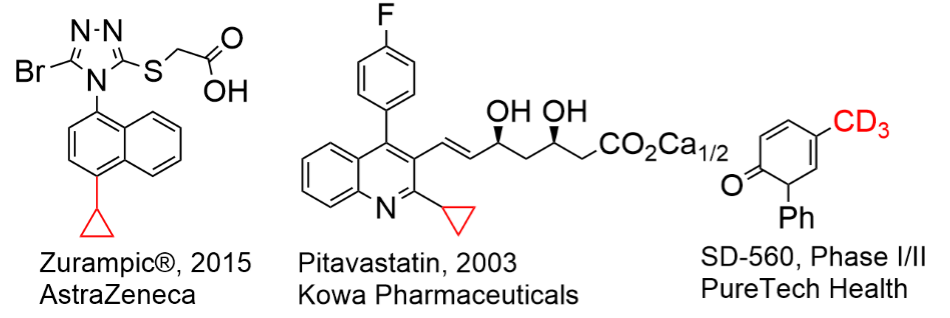
Scheme 2.Examples of drug molecules containing CD3 or cyclopropane fragment.
Our Csp2-Csp3 Coupling Technology on "Chemistry Informer Library" Molecules
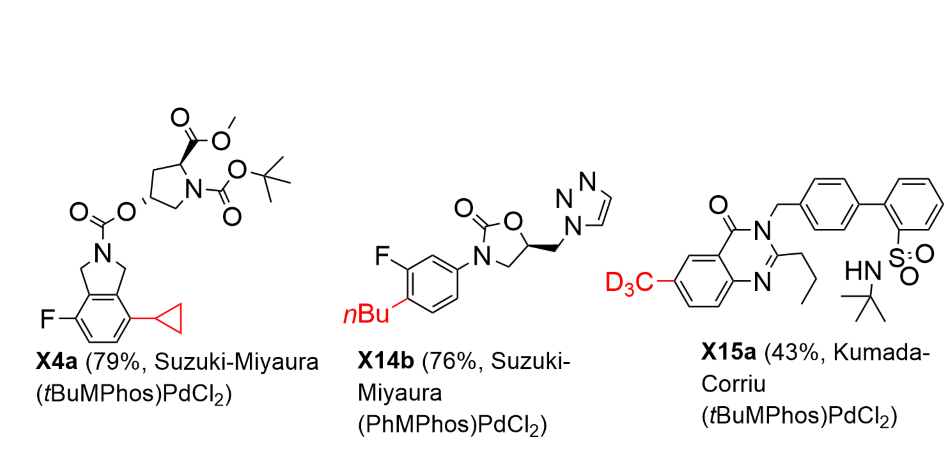
Scheme 3.Csp2-Csp3 couplings on “Chemistry Informer Library” molecules
References
To continue reading please sign in or create an account.
Don't Have An Account?