Hydrophilic Interaction Liquid Chromatography
The following pages contain an introduction to the HILIC separation technique and an overview of the strategies for developing robust methods for separation of polar and hydrophilic compounds.
What is HILIC?
Hydrophilic Interaction Liquid Chromatography
HILIC or Hydrophilic Interaction Liquid Chromatography is a high-performance liquid chromatographic (HPLC) technique for separation of polar and hydrophilic compounds. Originally the separation technique was called "Hydrophilic-Interaction Chromatography", and occasionally the expression "Aqueous Normal Phase" has also been used.
To put it simply: HILIC is a normal-phase type of separation but uses reversed-phase type eluents.
Thus, HILIC provides:
- A column with a hydrophilic stationary phase
- An eluent with water, buffer and a high concentration of water-miscible organic solvent.
A typical HILIC application uses acetonitrile at a concentration between 50-95% in an aqueous buffer such as ammonium formate, ammonium acetate or their acids, which have high solubility in organic solvents. HILIC can be used with many detection techniques, but when combined with electro spray ionisation mass spectrometry (ESI-MS) for example, HILIC will also enable higher sensitivities.
Reversed Elution Order Compared to RPLC
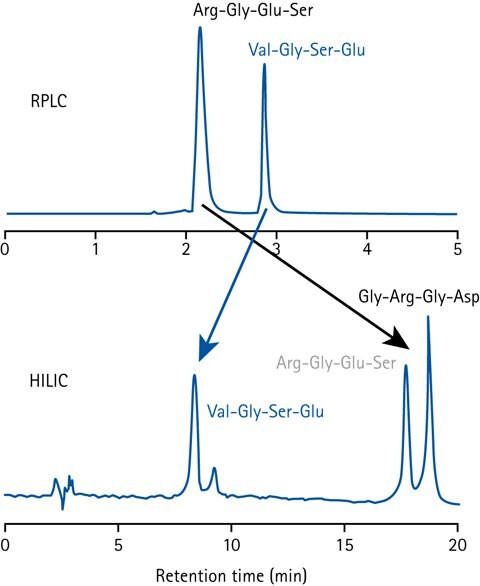
Figure 1.Comparison of peptide separation in HILIC and RPLC.
The elution order in HILIC is roughly the reverse of that in RPLC (Reversed-Phase Liquid Chromatography). A compound that elutes in the void volume on a RPLC column typically has high retention in HILIC, and vice versa.
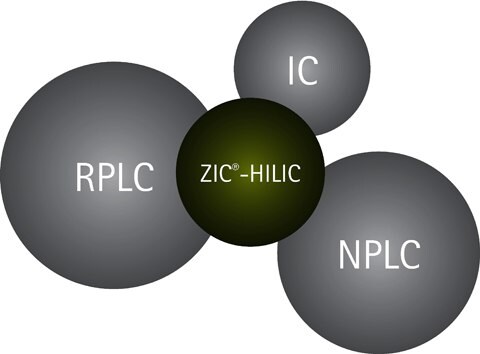
Figure 2.HILIC fills a gap in the chromatographic toolbox, but also partly overlaps with RPLC, NPLC, and IC separation techniques.
Compounds such as, acids, bases, ions, sugars, and other charged and neutral hydrophilic compounds that are troublesome to separate in RPLC, are much easier to separate in HILIC due to the different separation selectivity. Some compounds are possible to separate by more than one chromatographic technique, and HILIC does in that respect partly overlap both with RPLC, NPLC (Normal Phase Liquid Chromatography) and IC (Ion Chromatography)
Why Use HILIC?
Rapidly Increasing Interest for HILIC
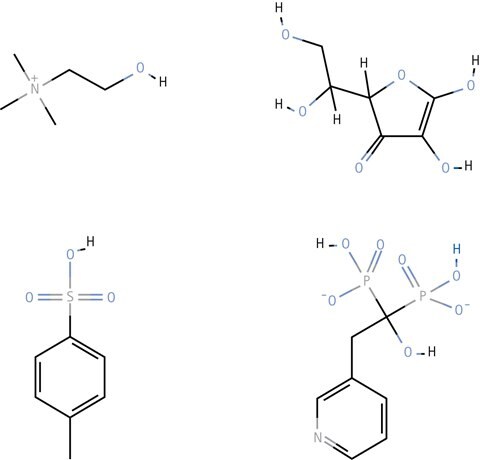
Figure 3.Example of compounds successfully separated using HILIC. Legend; Choline (top left), Ascorbic acid (top right), p-Toluenesulfonic acid (bottom left), and Risedronate (bottom right).
The HILIC separation technique is gaining much interest because it solves many of the previously difficult separation problems, including separation of small organic acids, basic drugs, cations, anions and many other neutral and charged substances.
To continue reading please sign in or create an account.
Don't Have An Account?