689017
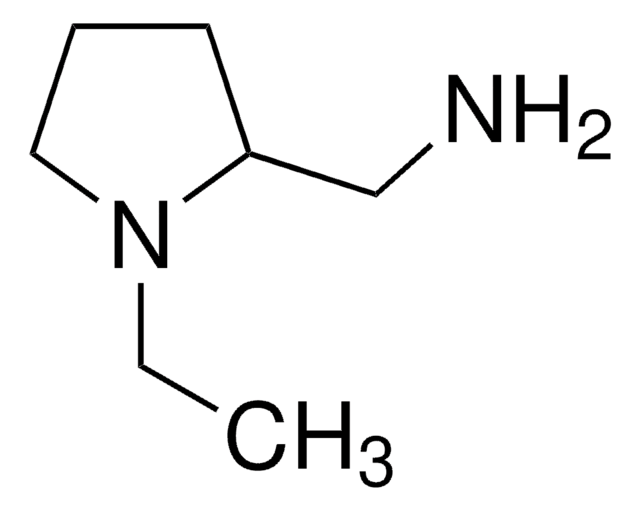
N-[(2S)-2-Pyrrolidinylmethyl]-trifluoromethanesulfonamide
≥98.5% (T)
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C6H11F3N2O2S
CAS Number:
Molecular Weight:
232.22
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98.5% (T)
form
powder
optical activity
[α]/D +13±1°, c = 1 in methanol
optical purity
ee: ≥98.5%
SMILES string
FC(F)(F)S(=O)(=O)NC[C@@H]1CCCN1
InChI
1S/C6H11F3N2O2S/c7-6(8,9)14(12,13)11-4-5-2-1-3-10-5/h5,10-11H,1-4H2/t5-/m0/s1
InChI key
RIWFUAUXWIEOTK-YFKPBYRVSA-N
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Liansuo Zu et al.
Organic letters, 8(14), 3077-3079 (2006-06-30)
[reaction: see text] A recycle and reusable fluorous (S)-pyrrolidine sulfonamide organocatalyst has been developed for promoting highly enantio- and diastereoselective Michael addition reactions of ketones and aldehydes with nitroolefins in water. The organocatalyst is conveniently recovered from the reaction mixtures
Direct, highly enantioselective pyrrolidine sulfonamide catalyzed Michael addition of aldehydes to nitrostyrenes.
Wei Wang et al.
Angewandte Chemie (International ed. in English), 44(9), 1369-1371 (2005-01-25)
Liansuo Zu et al.
Organic letters, 10(6), 1211-1214 (2008-02-15)
Fluorous (S) pyrrolidine sulfonamide serves as an efficient promoter for highly enantioselective aldol reactions of ketones and aldehydes with aromatic aldehydes on water. A notable feature of the organocatalyst is that it can be recovered from the reaction mixtures by
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service