569593
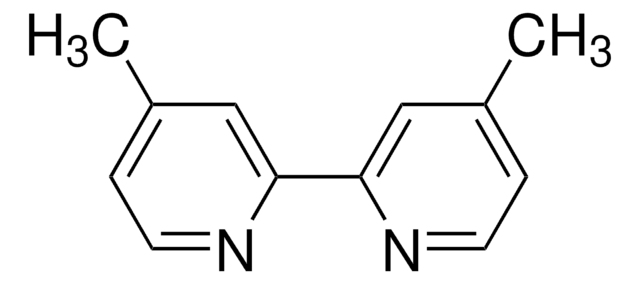
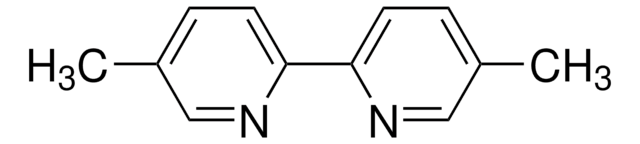
4,4′-Dimethyl-2,2′-dipyridyl
99.5%, purified by sublimation
Synonym(s):
2,2′-Bi(γ-picoline), 4,4′-Dimethyl-2,2′-bipyridine
About This Item
Recommended Products
Assay
99.5%
form
solid
purified by
sublimation
mp
169-174 °C (lit.)
SMILES string
Cc1ccnc(c1)-c2cc(C)ccn2
InChI
1S/C12H12N2/c1-9-3-5-13-11(7-9)12-8-10(2)4-6-14-12/h3-8H,1-2H3
InChI key
NBPGPQJFYXNFKN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- carbonato-bridged triangular trinuclear compounds and tetranuclear hydroxo-bridged compounds
- dmbipy-copper bridged complexes, which show variation in crystal geometries, thermal and magnetic properties
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Tools and techniques for performing atom transfer radical polymerization (ATRP) with benefits and limitations.
Arylboronic acids and esters are invaluable tools for the chemical community. These powerful reagents are used for a variety of transformations, most notably the Suzuki-Miyaura cross-coupling reaction.
We presents an article about a micro review of reversible addition/fragmentation chain transfer (RAFT) polymerization. RAFT (Reversible Addition/Fragmentation Chain Transfer) polymerization is a reversible deactivation radical polymerization (RDRP) and one of the more versatile methods for providing living characteristics to radical polymerization.
Applying ARGET ATRP to the Growth of Polymer Brush Thin Films by Surface-initiated Polymerization
Protocols
We presents an article featuring procedures that describe polymerization of methyl methacrylate and vinyl acetate homopolymers and a block copolymer as performed by researchers at CSIRO.
We present an article about RAFT, or Reversible Addition/Fragmentation Chain Transfer, which is a form of living radical polymerization.
An article about the typical procedures for polymerizing via ATRP, which demonstrates that in the following two procedures describe two ATRP polymerization reactions as performed by Prof. Dave Hadddleton′s research group at the University of Warwick.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service