92277
Trimethylamine N-oxide dihydrate
purum, ≥99.0% (NT)
Synonym(s):
TMANO
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
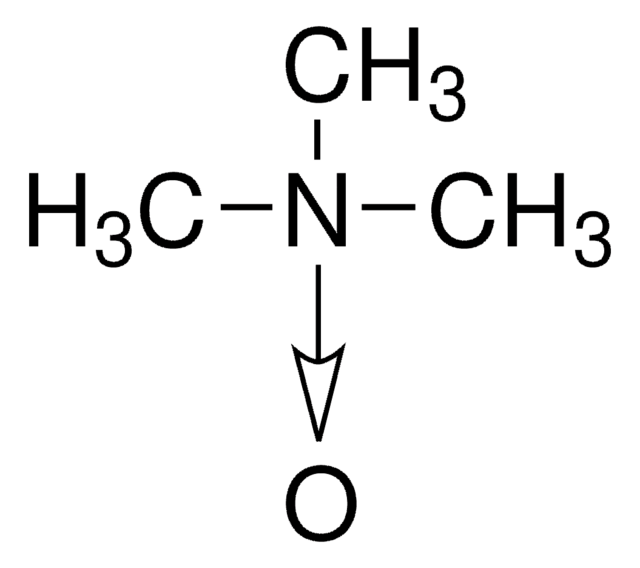
(CH3)3NO · 2H2O
CAS Number:
Molecular Weight:
111.14
Beilstein/REAXYS Number:
3612927
EC Number:
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
purum
Quality Level
assay
≥99.0% (NT)
form
crystals
reaction suitability
reagent type: oxidant
mp
95-99 °C (lit.)
95-99 °C
SMILES string
O.O.C[N+](C)(C)[O-]
InChI
1S/C3H9NO.2H2O/c1-4(2,3)5;;/h1-3H3;2*1H2
InChI key
PGFPZGKEDZGJQZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Trimethylamine N-oxide (TMAO) is an amphiphilic osmolyte that can counteract the denaturing effects of urea, pressure, and ice and stabilize the proteins.
Application
Reactant for:
- C-H bond cleavage
- Oxidation reactions (oxidant)
- Decarbonylating agent for solvent-free reactions
Trimethylamine N-oxide dihydrate can react with transition-metal carbonyl cluster compounds and activate CO displacement by converting CO ligands into carbon dioxide.
Other Notes
Oxidant for the catalytic OsO4 cis-hydroxylation of hindered olefins; preparation of the unstable, crystalline anhydrous compound
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Cluster synthesis. 10. Reaction of Os3 (CO) 10 (. mu. 3-S) with trimethylamine N-oxide dihydrate. Syntheses and structural characterizations of Os3 (CO) 8 (NMe3)(. mu.-OH)(. mu. 3-S)(. mu.-H) and the six-atom-chain cluster Os6 (CO) 18 (. mu.-OH)(. mu. 4-S)(. mu. 3-S)(. mu.-H).
Adams RD, et al.
Inorganic Chemistry, 25(8), 1122-1127 (1986)
Counteraction of urea by trimethylamine N-oxide is due to direct interaction.
Meersman F, et al.
Biophysical Journal, 97(9), 2559-2566 (2009)
J.A. Soderquist et al.
Tetrahedron Letters, 27, 3961-3961 (1986)
Trimethylamine N-oxide promoted reactions of manganese, molybdenum and tungsten carbonyl complexes.
Blumer DJ, et al.
Journal of Organometallic Chemistry, 173(1), 71-76 (1979)
Complex formation in aqueous trimethylamine-N-oxide (TMAO) solutions.
Hunger J, et al.
The Journal of Physical Chemistry B, 116(16), 4783-4795 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service