General description
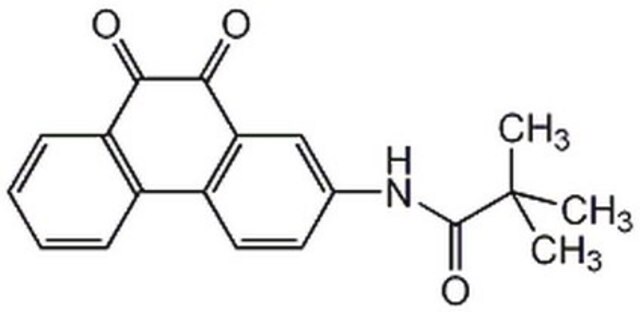
A cell-permeable chloronaphthoquinone compound that potently inhibits CD45/PTPRC tyrosine phosphatase activity (IC50 = 290 nM) in a non-substrate-competitive and irreversible manner via targeting an allosteric pocket at the D1-D2 domains interface. Exhibits good selectivity over MKPX, PRL-2, PTP1B/PTPN1, TC-PTP/PTPN2, SHP-1/PTPN6, PEP/PTPN22, LAR/PTPRF, and PTP-Sigma/PTPRS (IC50 >40 µM). Shown to reduce T cell receptor-mediated activation of Lck, Zap-70, MAPK, and IL-2 production in primary T cells and effectively block human Lck pY-394 dephosphorylation in Jurkat, but not CD45-deficient J45.01 line, reducing cellular metabolic activity without affecting viability. Displays immunosuppressive properties against footpad swelling in a murine modle of delayed type hypersensitivity among C57/BL6 mice even when administered (3 mg/kg, i.p) after early onset of inflammation. However, it does not clear existing edema when applied to mice with fully developed inflammation.
A cell-permeable chloronaphthoquinone that potently inhibits CD45/PTPRC tyrosine phosphatase activity (IC50 = 290 nM; substrate = 1 mM pp60-src pY-527 peptide) in a non-substrate-competitive, irreversible, and selective manner via targeting an allosteric pocket at the D1-D2 domains interface away from the substrate-binding/catalytic site, exhibiting little potency toward murine PEST or eight human phosphatases (IC50 >40 µM against pNPP hydrolysis by MKPX, PRL-2, PTP1B/PTPN1, TC-PTP/PTPN2, SHP-1/PTPN6, PEP/PTPN22, LAR/PTPRF, and PTP-Sigma/PTPRS). Shown to increase human Lck pY394 phosphorylation level in Jurkat cells (by 40% in 24 h; 0.5 µM), but not CD45-deficient J45.01 line. Likewise, drug treatment effectively suppresses induction of Lck pY394, Zap-70 pY319, pErk1/2 phosphorylations and IL-2 release in primary murine splenocyte cultures upon T-cell activation (0.5 µM for 48 h) via TCR cross-linking with concomitant reduced metabolic activity (by MTT assay), but not cell death (by Trypan Blue exclusion). Single intraperitoneal injection (3 mg/kg) administered either 1 h or 3 d post Ovalbumin-/OVA-challenge (1 µg /footpad) among OVA-sensitized mice effectively prevents footpad swelling 6 d post challenge (<0.05 mm vs. >0.3 mm, respectively, with or without treatment) in delayed type hypersensitivity (DTH) tests in vivo. Exhibits no detectable cytotoxicity in KB or HEK293 cultures (0.5 µM for 24 h), while a 50% lymphocytes reduction is reported in mice 24 h post 3 mg/kg i.p. dosage with no other apparent adverse effects.
Reconstitution
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 3 months at -20°C.
Use only fresh DMSO for reconstitution.
Other Notes
Perron, M., et al. 2014. Mol. Pharmacol.85, 553.