Recommended Products
form
powder
mol wt
average Mn ~70,000
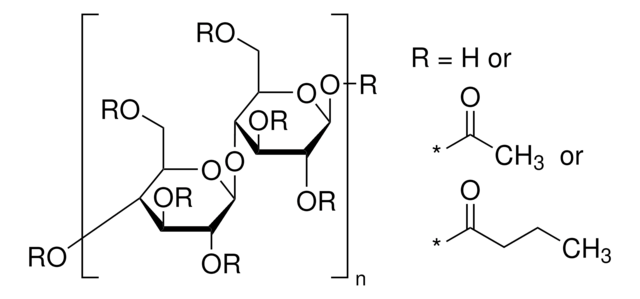
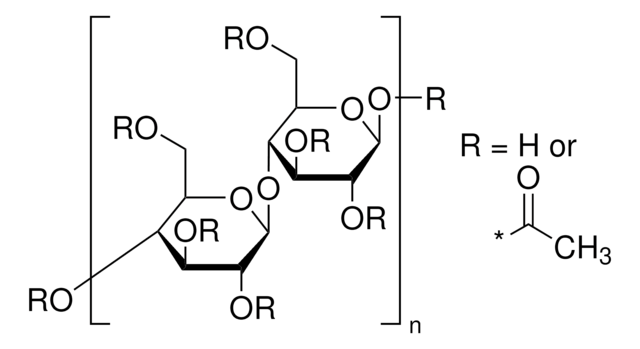
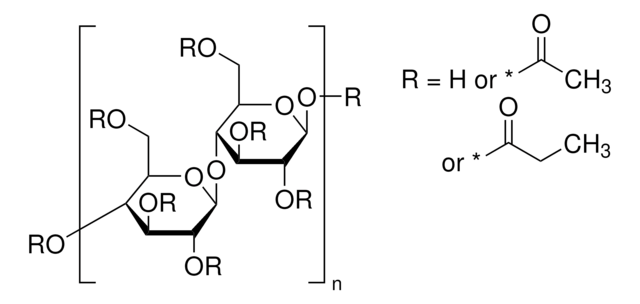
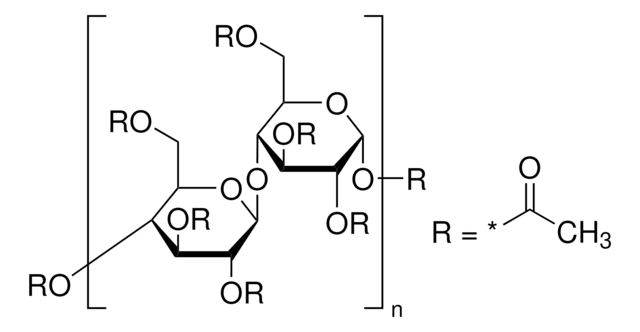
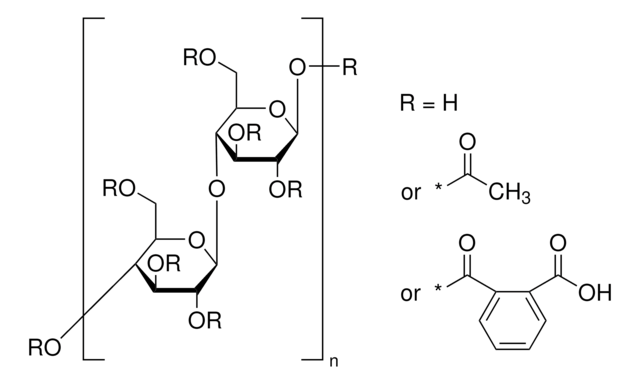
extent of labeling
12-15 wt. % Acetyl
35-39 wt. % Butyryl
1.2-2.2 wt. % Hydroxyl
refractive index
n20/D 1.475 (lit.)
density
1.25 g/mL at 25 °C (lit.)
Looking for similar products? Visit Product Comparison Guide
Storage Class
11 - Combustible Solids
wgk_germany
nwg
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
W M Obeidat et al.
Journal of microencapsulation, 22(3), 281-289 (2005-07-16)
The objectives of this investigation are to evaluate the encapsulation efficiency of the anti-thyroid agent 6-n-propyl-2-thiouracil using two polymers of different characteristics (cellulose acetate butyrate polymer, (CAB-551-0.01) and ammonio methacrylate copolymer (Eudragit RL 100) and to study the effect of
Min Soo Park et al.
Langmuir : the ACS journal of surfaces and colloids, 22(10), 4594-4598 (2006-05-03)
We investigate the effects of interfacial energy between water and solvent as well as polymer concentration on the formation of porous structures of polymer films prepared by spin coating of cellulose acetate butyrate (CAB) in mixed solvent of tetrahydrofuran (THF)
R B Umamaheshwari et al.
Drug delivery, 10(3), 151-160 (2003-08-29)
We prepared cellulose acetate butyrate (CAB)-coated cholestyramine microcapsules as a intragastric floating drug delivery system endowed with floating ability due to the carbon dioxide generation when exposed to the gastric fluid. The microcapsules also have a mucoadhesive property. Ion-exchange resin
Decheng Ma et al.
Journal of pharmaceutical and biomedical analysis, 35(4), 779-788 (2004-06-15)
The purpose of this study was to qualitatively and quantitatively determine potential cellulose acetate butyrate (CAB) extractables in a way to meaningfully predict the in vivo exposure resulting from clinical administration. Extractions of CAB-381-20 were performed in several solvent systems
W M Obeidat et al.
Journal of microencapsulation, 21(1), 47-57 (2004-01-14)
Theophylline microspheres were prepared by the emulsion-solvent evaporation method using cellulose acetate butyrate (CAB381-20) and mixtures of CAB381-20(R) and cellulose acetate phthalate. The physical state of the drug, polymers and microspheres surfaces were determined using scanning electron microscopy. For those
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service