PHR1375
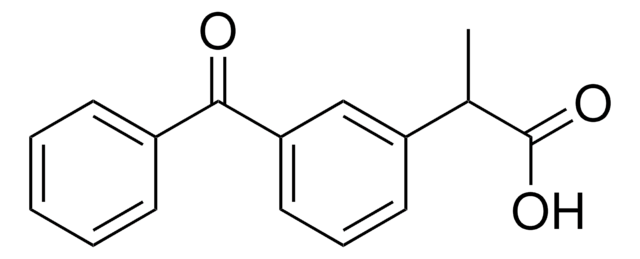
Ketoprofen
Pharmaceutical Secondary Standard; Certified Reference Material
Synonim(y):
Ketoprofen, KP, 2-(3-Benzoylphenyl)propionic acid
About This Item
Polecane produkty
klasa czystości
certified reference material
pharmaceutical secondary standard
Poziom jakości
agency
traceable to BP 668
traceable to Ph. Eur. K2000000
traceable to USP 1356632
rodzina API
ketoprofen
Certyfikat analizy
current certificate can be downloaded
metody
HPLC: suitable
gas chromatography (GC): suitable
Zastosowanie
pharmaceutical (small molecule)
format
neat
temp. przechowywania
2-30°C
ciąg SMILES
CC(C(O)=O)c1cccc(c1)C(=O)c2ccccc2
InChI
1S/C16H14O3/c1-11(16(18)19)13-8-5-9-14(10-13)15(17)12-6-3-2-4-7-12/h2-11H,1H3,(H,18,19)
Klucz InChI
DKYWVDODHFEZIM-UHFFFAOYSA-N
informacje o genach
human ... PTGS1(5742) , PTGS2(5743)
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Opis ogólny
Ketoprofen is an active pharmaceutical compound used as a non-steroidal anti-inflammatory drug (NSAID). It is essentially a non-narcotic drug extensively utilized for mild to moderate pain relief.
Zastosowanie
Działania biochem./fizjol.
Komentarz do analizy
Inne uwagi
Przypis
Polecane produkty
produkt powiązany
Hasło ostrzegawcze
Danger
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Acute Tox. 3 Oral - Aquatic Acute 1 - Eye Irrit. 2 - Skin Irrit. 2
Kod klasy składowania
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Klasa zagrożenia wodnego (WGK)
WGK 2
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Choose from one of the most recent versions:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej