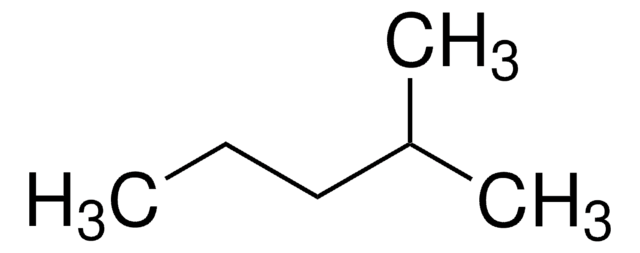
About This Item
Polecane produkty
klasa czystości
analytical standard
Poziom jakości
gęstość pary
2.97 (vs air)
ciśnienie pary
135 mmHg ( 17 °C)
Próba
≥99.5% (GC)
temp. samozapłonu
532 °F
okres trwałości
limited shelf life, expiry date on the label
granice wybuchowości
~7.7 %
metody
HPLC: suitable
gas chromatography (GC): suitable
współczynnik refrakcji
n20/D 1.376 (lit.)
n20/D 1.376
tw
64 °C (lit.)
gęstość
0.664 g/mL at 25 °C (lit.)
Zastosowanie
cleaning products
cosmetics
flavors and fragrances
food and beverages
personal care
format
neat
ciąg SMILES
CCC(C)CC
InChI
1S/C6H14/c1-4-6(3)5-2/h6H,4-5H2,1-3H3
Klucz InChI
PFEOZHBOMNWTJB-UHFFFAOYSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Zastosowanie
Polecane produkty
Hasło ostrzegawcze
Danger
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Aquatic Chronic 2 - Asp. Tox. 1 - Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Organy docelowe
Central nervous system
Kod klasy składowania
3 - Flammable liquids
Klasa zagrożenia wodnego (WGK)
WGK 2
Temperatura zapłonu (°F)
19.4 °F - closed cup
Temperatura zapłonu (°C)
-7 °C - closed cup
Środki ochrony indywidualnej
Eyeshields, Faceshields, Gloves
Choose from one of the most recent versions:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Protokoły
Separation of 4-Methyl-2-pentanone; Dimethyl disulfide; Hexanal; 3-Methylpentane; Acetone
Protocol for GC Analysis of Hydrocarbons in Gasoline on Petrocol® DH
-1,3-Dimethylcyclopentane; 1,1-Dimethylcyclopentane; 2,2,3-Trimethylpentane; 2,2-Dimethylbutane; 2,2-Dimethylhexane; 2,2-Dimethylpentane; 2,3-Dimethylbutane; 2,3-Dimethylhexane; 2,4-Dimethylheptane; 2,4-Dimethylpentane; 2,5-Dimethylheptane; 2-Methylhexane; 2-Methylpentane; 3,3-Dimethylpentane; 3,4-Dimethylhexane; 3-Ethylpentane; 3-Methyloctane; 4-Methylheptane; Ethylbenzene; Ethylcyclopentane; 2,6-Dimethylheptane; 3-Ethylheptane
-Xylene; Nonane; Propylbenzene; Mesitylene; 1,2,4-Trimethylbenzene; 1,2,3-Trimethylbenzene; 1,3-Diethylbenzene; 1,4-Dimethyl-2-ethylbenzene; 1,2-Dimethyl-4-ethylbenzene; Durene; 1,2,3,5-Tetramethylbenzene; 1,2,3,5-Tetramethylbenzene; 2-Methylnaphthalene (β)
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej